Abstract
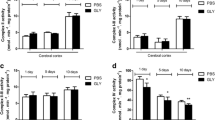
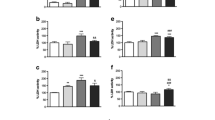
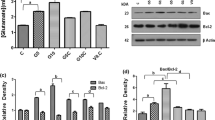
Glutamate, a major excitatory amino acid neurotransmitter is also an endogenous excitotoxin. The present study examined the prolonged and delayed effects of glutamate excitotoxicity on mitochondrial lipid peroxidation and antioxidant parameters in different brain regions, namely, cerebral hemisphere, cerebellum, brain stem and diencephalon. Wistar rats (male) were exposed to monosodium glutamate (MSG) (4 mg × g body wt−1, i.p.) for 6 consecutive days and sacrificed on 30th and 45th day after last MSG dose. MSG treatment markedly decreased the mitochondrial manganese superoxide-dismutase (Mn-SOD), catalase and reduced glutathione (GSH) content, and increased the lipid peroxidation (LPx), uric acid and glutathione peroxidase (GPx) activity. These results indicate that oxidative stress produced by glutamate in vulnerable brain regions may persist for longer periods and mitochondrial function impairment is an important mechanism of excitatory amino acid mediated neurotoxicity in chronic neurodegeneration.
Similar content being viewed by others
References
Nicholls DG, Budd SL: Mitochondria and neuronal survival. Physiol Rev 80: 315-360, 2000
Coyle JT, Puttfarcken P: Oxidative stress, glutamate, and neurodegenerative disorders. Science 262: 689-695, 1993
Keidrowski L, Costa E: Glutamate-induced destabilization of intracellular calcium concentration homeostasis in cultured cerebellar granule cells: Role of mitochondria in calcium buffering. Mol Pharmacol 47: 140-147, 1992
White RJ, Reynolds IJ: Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci 15: 1318-1328, 1995
Burgard EC, Hablitz JJ: N-Methyl-D-aspartate receptors-mediated calcium accumulation in neocortical neurons. Neuroscience 69: 351-362, 1995
Limbrick DD Jr, Churn SB, Sombati S, DeLorenzo RJ: Inability to restore resting intracellular calcium levels as an early indicator of delayed neuronal death. Brain Res 690: 145-156, 1995
Castilho RF, Hansson O, Ward MW, Budd SL, Nicholls DG: Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurosci 18: 10277-10286, 1998
Randall RD, Thayer SA: Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci 12: 1882-1895, 1992
Luetjens MC, Bui NT, Sengpiel B, Münstermann G, Poppe M, Krohn AJ, Bauerbach E, Krieglstein J, Prehn JHM: Delayed mitochondrial dysfunction in excitotoxic neuron death: cytochrome c release and a secondary increase in superoxide production. J Neurosci 20: 5715-5723, 2000
Castilho RF, Ward MW, Nicholls DG: Oxidative stress, mitochondrial function, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 72: 1394-1401, 1999
Dugan LL, Sensi SL, Canzoneiro LMT, Handran SD, Rothman SM, Lin TS, Goldberg MP, Choi DW: Mitochondrial production of reactive oxygen species in cortical neurons following exposure to N-methyl-D-aspartate. J Neurosci 15: 6377-6388, 1995
Reynolds IJ, Hastings TG: Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci 15: 536-537, 1995
Bindokas VP, Jordan J, Lee CC, Miller RJ: Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J Neurosci 16: 1324-1336, 1996
Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ: Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci 1: 366-373, 1998
Vergun O, Sobolevsky AI, Yelshansky MV, Keelan J, Khodorov BI, Duchen MR: Exploration of the role of reactive oxygen species in glutamate neurotoxicity in rat hippocampal neurons in culture. J Physiol 531: 147-163, 2001
Babu GN, Bawari M, Ali MM: Lipid peroxidation potential and antioxidant status of circumventricular organs of rat brain following neonatal monosodium glutamate. Neurotoxicol 15: 773-777, 1994
Bawari M, Babu GN, Ali MM, Mishra UK: Effects of neonatal monosodium glutamate on lipid peroxidation in adult rat brain. Neuro Report 6: 405-412, 1995
McCord JM, Fridovich I: Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049-6055, 1969
Aebi H: Catalase in vitro. In: L. Packer (ed). Methods in Enzymology, vol. 105. Academic Press, New York, 1984, pp 121-126
Sedlak J, Lindsay RH: Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25: 192-205, 1968
Flohe L, Gunzler WA: Assays of glutathione peroxidase. In: L. Packer (ed). Methods in Enzymology, vol. 105. Academic Press, New York, 1984, pp 114-121
Beuge JA, Aust AD: Microsomal lipid peroxidation. In: L. Packer (ed). Methods in Enzymology, vol. 52. Academic Press, New York, 1978, pp 302-310
Hsu C, Hsieh YL, Lue SI, Hsu HK: Sex specific expression of N-methyl-D-aspartate (NMDAR) in the preoptic area of neonatal rats. Neurosci Lett 262: 85-88, 1999
Kubo T, Kohira R, Okano T, Ishikawa K: Neonatal glutamate can destroy the hippocampal CA1 structure and impair discrimination learning in rats. Brain Res 616: 311-314, 1993
Olney JW: Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164: 719-721, 1969
Olney JW: Brain damage in infant mice following oral intake of glutamate, aspartate or cysteine. Nature 227: 600-601, 1970
Arees EA, Mayer J: Monosodium glutamate-induced brain lesions: Electron microscopic examination. Science 170: 549-550, 1970
Pizzi CJ, Saplosky R, Brahmbhat JE: Monosodium glutamate: Endocrinological effects on neonates. Pharmacol Biochem Behav 5: 551-557, 1976
Beal MF: Mechanism of excitotoxicity in neurologic diseases. FASEB J 6: 3338-3344, 1992
Beal MF: Role of excitotoxicity in human neurological diseases. Curr Opin Neurobiol 2: 657-662, 1992
Yoneda Y, Nobuyuki K, Kitayama T, Hinoi E: Consolidation of transient ionotropic glutamate signals through nuclear transcription factors in the brain. Prog Neurobiol 63: 697-719, 2001
Zhu CH, Huang Y, Oberley LW, Domann FE: A family of AP-2 proteins down-regulate manganese superoxide dismutase expression. J Biol Chem 276: 14407-14413, 2001
Kono Y, Fridovich I: Superoxide radical inhibits catalase. J Biol Chem 257: 5751-5754, 1982
Patel M, Day BJ, Crapo JD, Fridovich I, McNamara JO: Requirements for superoxide in excitotoxic cell death. Neuron 16: 345-355, 1996
Gardner PR, Fridovich I: Superoxide sensitivity of Escherichia coli 6-phosphogluconate dehydrogenase. J Biol Chem 266: 1478-1483, 1991
Qing-You L, Pederson C, Day BJ, Patel M: Dependence of excitotoxicity neurodegeneration on mitochondrial aconitase inactivation. J Neurochem 78: 746-755, 2001
Doroshow JH, Locker GY, Myers CE: Enzymatic defenses of the mouse heart against reactive oxygen metabolites: Alterations produced by doxorubicin. J Clin Invest 65:128-135, 1980
Kakkar R, Kalra J, Mantha SV, Prasad K: Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 151: 113-119, 1995
Bhardwaj SK, Sharma P, Kaur G: Alterations in free radical scavenger system profile of type I diabetic rat brain. Mol Chem Neuropathol 35: 187-202, 1998
Halliwell B: Oxidant and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson's disease, Alzheimer's disease, traumatic injury or stroke? Acta Neurol Scand Suppl 126: 23-33, 1989
Babu GN, Bawari M: Single microinjection of L-glutamate induces oxidative stress in discrete regions of rat brain. Biochem Mol Biol Int 43: 1207-1217, 1997
Bhardwaj SK, Sharma ML, Gulati G, Chabbra A, Kaushik R, Sharma P, Kaur G: Effect of starvation and insulin induced hypoglycemia on oxidative stress scavenger system and electron transport chain complexes from rat brain, liver and kidney. Mol Chem Neuropathol 34: 157-168 1998
Chen LH: Interaction of vitamin E and ascorbic acid. In Vivo 3: 199-210, 1989
Retsky SC, Frei B: Vitamin C prevents metal ion-dependent initiation and propagation of lipid peroxidation in human low-density lipoprotein. Biochim Biophys Acta 1257: 279-287, 1995
Dawson VL, Dawson TM, London Ed, Bredt DS, Snyder SH: Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 88: 6368-6371, 1991
Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA: Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartatae or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA 92: 7162-7166, 1995
Budd SL, Nichollas DG: A reevaluation of the role of mitochondria in neuronal calcium homeostasis. J Neurochem 66: 403-411, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Singh, P., Arora Mann, K., Kaur Mangat, H. et al. Prolonged glutamate excitotoxicity: Effects on mitochondrial antioxidants and antioxidant enzymes. Mol Cell Biochem 243, 139–145 (2003). https://doi.org/10.1023/A:1021668314070
Issue Date:
DOI: https://doi.org/10.1023/A:1021668314070