Abstract
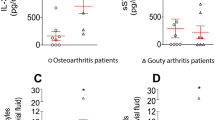
Superoxide anion (O2°-)production by neutrophil NADPH oxidase participates in arthritic joint lesion formation. Proinflammatory cytokines such as tumor necrosis factor α(TNFα), interleukin 8 (IL-8) and granulocyte/macrophage-colony stimulating factor (GM-CSF) have a priming effect on neutrophil NADPH oxidase activity. NADPH oxidase activation is dependent on phosphorylation of p47phox, a cytosolic component of the enzyme. We studied O2°-production and p47phox phosphorylation in synovial fluid (SF) from patients with rheumatoid arthritis (RA) and spondylarthropathy (SpA) according to TNFα, IL-8 and GM-CSF levels. O2°-production by neutrophils isolated from SF of all the arthritis patients (RA and SpA) was higher than that of circulating resting neutrophils and when stimulated with fMLP or PMA. In addition, p47phox was partially phosphorylated in SF neutrophils compared to circulating neutrophils. High levels of TNFα and IL-8 (but not GM-CSF) are detected in patient's SF (compared to circulating blood levels). TNFα levels were significantly higher in RA than in SpA SF. These results suggest that increased NADPH oxidase activity could be involved in arthritic joint inflammation through increased p47phox phosphorylation. This could be the result of the presence of high levels of priming agents such as TNFα and IL-8 but not GM-CSF.
Similar content being viewed by others
REFERENCES
Firestein, G. S. and N. J. Zvaifler. 1992. Rheumatoid Arthritis: a disease of disordered immunity. In: Inflammation, Basic principles and clinical correlates, Second edition, J. I. Gallin and I. M. Goldstein, ed: Raven Press, New York, p. 959–977.
Babior, B.M. 1984. Oxidants from phagocytes: agents of defense and destruction. Blood 64:959–966.
Smith, J. A. 1994. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukoc. Biol. 56:672-686.
Hallett, M.B. and D. L. Lloyds. 1995. Neutrophil priming: the cellular signals that say amber but not green. Immunology Today 16:264-268.
Elbim, C., S. Bailly, S. Chollet-Martin, J. Hakim, and MA. Gougerot-Pocidalo. 1994. Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect. Immun. 62:2195–2201.
Lambeth, J.D. 2000. Regulation of phagocyte respiratory burst oxidase by protein interactions. J. Biochem. Mol. Biol. 33:427-439.
Chanock, S.J., J. El Benna, R. M. Smith, and B. M. Babior. 1994. The respiratory burst oxidase. J. Biol. Chem. 269:24519–24522.
DeLeo, F.R. and M. T. Quinn. 1996. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J. Leukoc. Biol. 60:677–691.
Segal, A.W., P. G. Heyworth, S. Cockroft, and M. M. Barrowman. 1985. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature 316:547–549.
El Benna, J., P. M. C. Dang, M. Gaudry, and et al. 1997. Phosphorylation of the respiratory burst oxidase subunit p67phox during human neutrophil activation. Regulation by protein kinase C-dependent and independent pathways. J. Biol. Chem. 272:17204–17208.
Rotrosen, D. and T. L. Leto. 1990. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. Translocation to membrane is associated with distinct phosphorylation events. J. Biol. Chem. 265:19910–19915.
El Benna, J., L. P. Faust, and B. M. Babior. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 269:23431–234316.
Faust, L. P., J. El Benna, B. M. Babior, ans S. J. Chanock SJ. 1995. The phosphorylation targets of p47phox, a subunit of the respiratory burst oxidase. Functions of the individual target serines as evaluated by site-directed mutagenesis. J. Clin. Invest. 96:1499–1505.
Dang, P. M. C., C. Dewas, M. Gaudry, and et al. 1999. Priming of human neutrophil respiratory burst by granulocyte ?macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47phox. J. Biol. Chem. 274:20704–20708.
Arnett, F. C., S. M. Edworthy, D. A. Bloch, and et al. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315–324.
Dougados, M., S. Van der Linden, R. Juhlin, and et. al. 1991. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 34:1218-1227.
Ramey, D. R., J. Raynauld, and J. F. Fries. 1992. The Health Assessment Questionnaire Arthritis Care Res. 5:119–129.
Prevoo, M. L. L., M. A. van't Hof, H. H. Kuper, M. A. van Leeuven, L. B. A. van de Putte, and P. L. C. M. van Riel. 1995. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 38:44–48.
Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Towbin, H., T. Staehlin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350–4354.
Eggleton, P., L. Wang, J. Penhallow, N. Crawford, and K. A. Brown. 1995. Differences in oxidative response of subpopulations of neutrophils from healthy subjects and patients with rheumatoid arthritis. Ann. Rheum. Dis. 54:916–923.
Weisbart, R.H., K. L., D. W. Golde, S. C. Clark, G. G. Wong, and J. C. Gasson. 1987. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood 69:18–21.
Taylor PC. 2001. Anti-tumor necrosis factor therapies. Curr. Opin. Rheumatol. 13(3):164–169.
Lloyds, D., E. V. Davies, B. D. Williams, and M. B. Hallett. 1996. Tyrosine phosphorylation in neutrophils from synovial fluid of patients with rheumatoid arthritis. Brit. J. Rheumatol. 35:846–852.
Dusi, S., V. Della Bianca, M. Donini, K. A. Nadalini, and R. Rossi. 1996. Mechanisms of stimulation of the respiratory burst by TNF in nonadherent neutrophils: its independence of lipidic transmembrane signaling and dependence on protein tyrosine phosphorylation and cytoskeleton. J. Immunol. 157:4615–4623.
Yuo, A., E. Okuma, S. Kitagawa, and F. Takaku. Tyrosine phosphorylation of p38 but not extracellular signal-regulated kinase in normal neutrophils stimulated by tumor necrosis factor: comparative study with granulocyte-macrophage colony-stimulating factor. Biochem. Biophys. Res. Comun. 235:42–46.
Al-Shami, A. and P. H. Naccache. 1999. Granulocyte-macrophage colony-stimulating factor-activated signaling pathways in human neutrophils. Involvement of Jak2 in the stimulation of phosphatidylinositol 3-kinase. J. Biol. Chem. 274:5333–5338.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Benna, J.E., Hayem, G., Dang, P.MC. et al. NADPH Oxidase Priming and p47phox Phosphorylation in Neutrophils from Synovial Fluid of Patients with Rheumatoid Arthritis and Spondylarthropathy. Inflammation 26, 273–278 (2002). https://doi.org/10.1023/A:1021460517468
Issue Date:
DOI: https://doi.org/10.1023/A:1021460517468