Abstract
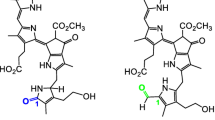
The demethoxycarbonyl reaction of pheophorbide a in plants and algae was investigated. Two types of enzyme that catalyze alternative reactions in the formation of pyropheophorbide a were found. One enzyme, designated `pheophorbidase (Phedase)', was purified nearly to homogeneity from cotyledons of radish (Raphanus sativus). This enzyme catalyzes the conversion of pheophorbide a to a precursor of pyropheophorbide a, C-132-carboxylpyropheophorbide a, by demethylation, and then the precursor is decarboxylated non-enzymatically to yield pyropheophorbide a. The activity of Phedase was inhibited by the reaction product, methanol. The other enzyme, termed `pheophorbide demethoxycarbonylase (PDC)', was highly purified from the Chl b-less mutant NL-105 of Chlamydomonas reinhardtii. This enzyme had produced no intermediate as shown in the Phedase reaction, indicating that it converts pheophorbide a directly into pyropheophorbide a, probably by nucleophilic reaction. Phedase and PDC consisted of both senescence-induced and constitutive enzymes. The molecular weight of both Phedases was 113 000 and of senescence-induced PDC was 170 000. The K m values against pheophorbide a for both Phedases were 14–15 μM and 283 μM for senescence-induced PDC. The activity of both Phedases was inhibited by the reaction product, methanol, whereas methanol had no specific effect on senescence-induced PDC. Phenylmethylsulfonic fluoride and N-ethylmaleimide inhibited the senescence-induced Phedase and PDC, respectively. Among the 23 species from 15 different families tested, Phedase activity was found in 10 species from three families. PDC activity was not detected in plants lacking Phedase activity, except for Chlamydomonas. Based on these findings, a likely conclusion is that at least two alternative pathways that are catalyzed by two different enzymes, Phedase and PDC, exist for the formation of pyropheophorbide a.
Similar content being viewed by others
References
Azuma R, Takahashi Y, Kurata H, Kawano T, Shimokawa K and Adachi M (1999) Does peroxidase act as a 'Mg-dechelatase'? Plant Peroxidase Newslett 13: 145–151
Doi M, Shima S, Egashira T, Nakamura K and Okayama S (1997) New bile pigment excreted by a Chlamydomonas reinhardtii mutant: a possible breakdown catabolite of chlorophyll a. J Plant Physiol 150: 504–508
Doi M, Inage T and Shioi Y (2001) Chlorophyll degradation in a Chlamydomonas reinhardtii mutant: an accumulation of pyropheophorbide a by anaerobiosis. Plant Cell Physiol 42: 469–474
Haidl H, Knödlmayr K, Rüdiger W, Scheer H, Schoch S and Ullrich J (1985) Degradation of bacteriochlorophyll a in Rhodopseudomonas sphaeroides R26. Z Naturforsch 40c: 685–692
Kurata H, Maeda Y, Adachi M and Shimokawa K (1998) A possible participation of pyropheophorbide a in chlorophyll catabolism during ripening of Citrus unshiu fruits. J Japan Soc Hort Sci 67: 651–654
Langmeier M, Ginsburg S and Matile P (1993) Chlorophyll breakdown in senescent leaves: demonstration of magnesium-dechelatase activity. Physiol Plant 89: 347–353
Mach JM, Castillo AR, Hoogstraten R and Greenberg JT (2001) The Arabidopsis-accelerated cell death gene ACD2 encodes red chlorophyll catabolite reductase and suppresses the spread of disease symptoms. Proc Natl Acad Sci USA 98: 771–776
Matile P, Hörtensteiner S and Thomas H (1999) Chlorophyll degradation. Annu Rev Plant Physiol Plant Mol Biol 50: 67–95
Oberhuber M, Berghold J, Mühlecker W, Hörtensteiner S and Kräutler B (2001) Chlorophyll breakdown-on a nonfluoresent chlorophyll catabolite from spinach. Helv Chim Acta 84: 2615–2627
Owens TG and Falkowski PG (1982) Enzymatic degradation of chlorophyll a by marine phytoplankton in vitro. Phytochemistry 21: 979–984
Schoch S and Vielwerth FX (1983) Chlorophyll degradation in senescent tobacco cell culture (Nicotiana tabacum var. 'Sumsun'). Z Pflanzenphysiol 110: 309–317
Schoch S, Scheer H, Schiff JA, Rüdiger W and Siegelman HW (1981) Pyropheophytin a accompanies pheophophytin a in darkened light grown cells of Euglena. Z Naturforsch 36c: 827–833
Shimokawa K, Hashizume A and Shioi Y (1990) Pyropheophorbide a, a catabolite of ethylene-induced chlorophyll a degradation. Phytochemistry 29: 2105–2106
Shioi Y, Tatsumi Y and Shimokawa K (1991) Enzymatic degradation of chlorophyll in Chenopodium album. Plant Cell Physiol 32: 87–93
Shioi Y, Watanabe K and Takamiya K (1996) Enzymatic conversion of pheophorbide a to the precursor of pyropheophorbide a in leaves of Chenopodium album. Plant Cell Physiol 37: 1143–1149
Suzuki Y and Shioi Y (1999) Detection of chlorophyll breakdown products in the senescent leaves of higher plants. Plant Cell Physiol 40: 909–915.
Suzuki Y and Shioi Y (2001) Degradation of chlorophylls: two reaction pathways in the formation of pyropheophorbide a. PS2001 Proceedings (12th International Congress of Photosynthesis), S2–021. CSIRO Publishing, Collingwood, Australia
Takamiya K, Tsuchiya T and Ohta H (2000) Degradation pathway(s) of chlorophyll: what has gene cloning revealed? Trends Plant Sci 5: 426–431
Tsuchiya T, Ohta H, Okawa K, Masuda T, Mikami B, Kita N, Shioi Y and Takamiya K (1997) Purification and characterization of two isozymes of chlorophyllase from mature leaves of Chenopodium album. Plant Cell Physiol 38: 1026–1031
Watanabe K, Tsuchiya T, Masuda T, Ohta H, Takamiya K and Shioi Y (1995) Characterization and some properties of pheophorbidase from Chenopodium album. In: Mathis P (ed) Photosynthesis: from Light to Biosphere, Vol 3, pp 981–984. Kluwer Academic Publishers, Dordrecht, The Netherlands
Watanabe K, Ohta H, Tsuchiya T, Mikami B, Masuda T, Shioi Y and Takamiya K (1999) Purification and some properties of pheophorbidase in Chenopodium album. Plant Cell Physiol 40: 104–108
Ziegler R, Blaheta A, Guha N and Schönegge B (1988) Enzymatic formation of pheophorbide and pyropheophorbide during chlorophyll degradation in amutant of Chlorella fusca Shihira et Kraus. J Plant Physiol 132: 327–332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suzuki, Y., Doi, M. & Shioi, Y. Two enzymatic reaction pathways in the formation of pyropheophorbide a . Photosynthesis Research 74, 225–233 (2002). https://doi.org/10.1023/A:1020919929608
Issue Date:
DOI: https://doi.org/10.1023/A:1020919929608