Abstract
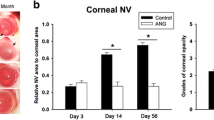
Lipid hydroperoxides (LHP) at high concentrations are cytotoxic, but at sublethal concentration, they induce synthesis of cytokine vascular growth factors. Intracorneal injections of 30 μg LHP placed 5 mm from the superior limbus stimulated early vasodilation of limbal vasculature and a rapidly developing, sustained neovascularization. Under these conditions, vessels grew at the rate of 0.3 mm/day to a total length of 7.5 mm, 25 days after injection. Cholesterol peroxides were less effective. Developing vessels were oriented towards the stimulus. Around the developing vessel there was dissolution of the stromal extracellular matrix. The most distal endothelial cells displayed prominent endoplasmic reticulum, a lack of basement membrane or tight junction complexes and leakage of fluorescein dye. Both the injection site and superior quadrant showed increased levels of tumor necrosis factor (TNF)-α and vascular endothelial growth factor after exposure to LHP. The neovascular response was inhibited by simultaneous administration of TNF-α antibody or pentoxifylline, an inhibitor of TNF-α synthesis. This corneal model of peroxide-induced neovascularization should prove useful for temporal studies of events in the initiation and propagation of signals leading to neovascularization, and for evaluating effects of treatment on neovascular growth.
Similar content being viewed by others
References
Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267, 10931–10934.
Hockel M, Schlenger K, Doctrow S, Kissel T, Vaupel P. Therapeutic angiogenesis. Arch Surg 1993; 128, 423–429.
Folkman J. Tumor angiogenesis. In: Holland JF, Free III E, Bast Jr RC, Kufe DW, Morton DL, Weichselbaum RR (eds), Cancer Medicine. Philadelphia: Lea & Febiger 1993; 1, 153–170.
Williamson JR, Chang K, Frangos M, et al. Hyperglycaemic pseudohypoxia and diabetic complications. Diabetes 1993; 42, 801–813.
Kunisaki M, Umeda F, Muchi T, Masakado M, Nawata H. High glucose reduces specific binding for d-α tocopherol in cultured aortic endothelial cells. Diabetes 1993; 42, 1138–1146.
Wolf SP. The potential role of oxidation and its complications: novel implications for theory and therapy. In: Crabbe MD (ed.), Diabetic Complications: Scientific and Clinical Aspects. London: Churchill Livingstone 1987: 167–220.
Armstrong D, Abdella N, Salman A, Miller N, Abdel-Rahman E, Bojancyzk M. Relationship of lipid peroxides to diabetic complications: comparison with conventional laboratory tests. J Diab Comp 1992; 6, 116–122.
Gallou G, Ruelland A, Campion L, Allannic H, Legras, B, Cloarec L. Susceptibility of LDL to lipid peroxidation in non-insulin dependent diabetes mellitus with or without macroangiopathy. Ann Biol Clin (Paris) 1994; 52, 695–699.
Haffner SM, Agil A, Mykkanen L, Stern MP, Jialal I. Plasma oxidizability in subjects with glucose intolerance, impaired glucose tolerance, and NIDDM. Diabetes Care 1995; 18, 646–653.
Armstrong D, Al-Awadi F. Lipid peroxidation and retinopathy in streptozotocin-induced diabetes. Free Rad Biol Med 1991; 11, 433–436.
Kowluru RA, Kern TS, Engerman RL, Armstrong D. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. III. Effects of antioxidants. Diabetes 1996; 45, 1233–1237.
Robison WG Jr, Laver NM, Jacot JL, Glover JP. Sorbinol prevention of diabetic-like retinopathy in the galactose-fed rat model. Invest Ophthalmol Vis Sci 1995; 36, 2368–2380.
Kador PF, Takahashi Y, Wyman M, Ferris F III. Diabetes like proliferative retinal changes in glactose-fed dogs. Arch Ophthalmol 1995; 113, 352–354.
Engerman RL, Kern TS. Retinopathy in galactose dogs continues to progress after cessation of galactosemia. Arch Ophthalmol 1995; 113, 355–358.
Armstrong D, Ueda T, Ueda T, et al. Lipid hydroperoxide stimulates retinal neovascularization in rabbit cornea through expression of tumor necrosis factor-α, vascular endothelial growth factor and platelet-derived growth factor. Angiogenesis 1998; 2, in press.
Klintworth GK. Corneal Angiogenesis. A Comprehensive Critical Review. Berlin: Springer Verlag 1990.
Armstrong D, Hiramitsu T, Gutteridge J, Nilsoon SE. Studies on experimentally induced retinal degeneration. Effect of lipid peroxides on electroretinographic activity in albino rabbits. Exp Eye Res 1982; 35, 157–171.
Korytowski W, Bachowski G, Girotti A. Chromatographic separation and electrochemical determination of cholesterol hydroperoxides generated by photodynamic action. Anal Biochem 1991; 197, 149–156.
Stefanini M, DeMartino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature 1967; 216, 173–174.
Armstrong D, Browne R. The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Adv Exp Biol Med 1994; 366, 43–58.
Ignatowski T, Spengler R. Tumor necrosis factor alpha: presynaptic sensitivity is modified after antidepressant drug administration. Brain Res 1994; 665, 293–299.
Nassif X, Mathison JC, Wolfson E, Koziol JA, Ulevitch RJ, So M. Tumor necrosis factor alpha antibody protects against lethal meningococcaemia. Mol Microbiol 1992, 6, 591–597.
Langham M. Observations on the growth of blood vessels into the cornea. Application of a new experimental technique. Br J Ophthalmol 1953; 35, 718.
Ausprunk DH, Folkman J. Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 1977; 14, 53–65.
Li NW, Grayson G, Folkman J, D'Amore PA. Sustained release endotoxin. A model for inducing corneal neovascularization. Invest Ophthalmol Vis Sci 1991; 32, 2906–2911.
Loughnan MS, Chatzistefanon K, Houzalez EM, et al. Experimental corneal neovascularization using sucralfate and basic fibroblast growth factor. Aust NZ J Ophthalmol 1995; 24, 289–295.
Proia AD, Hirakata A, McInnes JS, Scroggs MW, Parikh I. The effect of angiostatic steroids and beta cylodextrin tetradecasulfate on corneal neovascularization in the rat. Exp Eye Res 1993; 57, 693–698.
Koch AE, Cho M, Burrows JC, Polverini PJ, Leibovich SJ. Inhibition of production of monocyte/macrophage derived angiogenic activity by oxygen free radical scavengers. Cell Biol Int 1992; 16, 415–425.
Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci 1996; 37, 1625–1632.
Fukuda S, Alada A, Spengler R, et al. Angiogenic factor response of rabbit corneal stroma cells to lipid hydroperoxide stress in vitro. Invest Ophthalmol Vis Sci 1996; 37, 1014 (ARVO abstr).
Strieter RM, Remick DG, Ward PA, et al. Cellular and molecular regulation of tumor necrosis factor alpha production by pentoxifylline. Biochem Biophys Res Commun 1988; 155, 1230–1236.
Leibovich SJ, Polverini, PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 1987; 329, 630–632.
Hu DE, Hori Y, Presta M, Gresham GA, Fan T-P. Inhibition of angiogenesis by IL-1 receptor antagonist and selected cytokine antibodies. Inflammation 1994; 18, 45–58.
Friedlander M, Brooks PC, Shaffer RW, et al. Definition of two angiogenic pathways by distinct × integrins. Science 1995; 270, 1500–1502.
Hunt JV, Wolff SP. Oxidative glycation and free radical production: a causal mechanism of diabetic complications. Free Rad Res Commun 1991; 12/13, 115–123.
Maurice DM, Riley MV. The cornea; cellular and miscellaneous components. In: Graymore CN (ed.), Biochemistry of the Eye. New York: Academic Press 1970: 86–94.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ueda, T., Ueda, T., Fukuda, S. et al. Lipid hydroperoxide-induced tumor necrosis factor (TNF)-α, vascular endothelial growth factor and neovascularization in the rabbit cornea: effect of TNF inhibition. Angiogenesis 1, 174–184 (1997). https://doi.org/10.1023/A:1018377621102
Issue Date:
DOI: https://doi.org/10.1023/A:1018377621102