Abstract
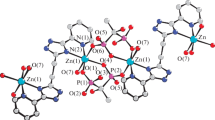
Two novel six-coordinated zinc complexes, tetra-aqua(1,10-phenanthroline)zinc dinitrate monohydrate, [Zn(H2O)4(phen)](NO3)2·H2O (1) and aqua(2,2′-bipyridine)(imidazole)-nitratozinc nitrate, [ZnNO3(H2O)(bipy)(Him)]NO3 (2) were prepared. Compound 1 crystallizes in the monoclinic space group P21/c with a = 8.780(4), b = 13.609(4), c = 15.368(5) Å, β = 93.86(2)°C, V = 1832.1(12) Å3. In 1, a phenanthroline molecule chelates the zinc atom and four water molecules complete the octahedral geometry around the metal. Compound 2 crystallizes in the monoclinic space group P21/n with a = 7.250(1), b = 24.554(4), c = 10.258(2) Å, β = 107.880(10)°, V = 1737.9(5) Å3. In 2, a bipyridine molecule and a nitrate anion chelate the zinc atom. An imidazole molecule and a water molecule then complete the six-coordinated geometry around zinc. The intermolecular packing is controlled by hydrogen bonding, especially in 1 and by π stacking. The thermal stability of the compounds and the loss of water molecules and ligands was monitored by a thermogravimetric study.
Similar content being viewed by others
References
Barton, J.K. Pure Appl. Chem. 1989, 61, 563.
Burley, S.K.; David, P.R.; Sweet, R.M.; Taylor, A.; Lipscomb, W.N. J. Mol. Biol. 1992, 124, 113.
Müller-Hartmann, A.; Vahrenkamp, H. Eur. J. Inorg. Chem. 2000, 2355.
Strager, N.; Lipscomb, W.N.; Klabunde, T.; Krebs, B. Angew. Chem., Int. Ed. Engl. 1996, 35, 2024.
Zhang, C.; Du, W.; Mei, Y. J. Chem. Res., Synop. 2000, 214.
Chen, X.M.; Xu, Z.T.; Huang, X.C. J. Chem. Soc., Dalton Trans. 1994, 2331.
Vallee, B.L.; Auld, D.S. Acc. Chem. Res. 1993, 26, 543.
Chang, S.; Karambelkar, V.V.; diTargiani, R.C.; Goldberg, D.P. Inorg. Chem. 2001, 40, 194.
Lipscomb, W.N.; Strager, N. Chem. Rev. 1996, 96, 2375.
Uhlenbrock, S.; Krebs, B. Angew. Chem., Int. Ed. Engl. 1992, 31, 1451.
Zhang, C.; Yu, K.; Wu, D.; Zhao, C. Acta Crystallogr., Sect. C, 1999, 55, 1815.
Zhang, C.; Yu, K.; Wu, D.; Zhao, C. Acta Crystallogr., Sect. C, 1999, 55, 1470.
Sheldrick, G.M. SHELXS-97, Programs for the Solution of Crystal Structures; University of Göttingen: Germany, 1997.
Sheldrick, G.M. SHELXL-97, Programs for the Refinement of Crystal Structures; University of Gottingen: Germany, 1997.
(a) Burnett, M.N.; Johnson, C.K. ORTEP-III: Oak Ridge Thermal Ellipsoid Plot Program for Crystal Structure Illustrations; Report ORNL-6895; Oak Ridge National Laboratory: Oak Ridge, TN, 1996; (b) Farrugia, L.J. ORTEP-III for Windows, version 1.0.1β; University of Glasgow, 1997; (c) Farrugia, L. J. J. Appl. Crystallogr. 1997, 30 565.
(a) Spek, A.L. Acta Crystallogr. Sect. A 1990, 46, C34; (b) Farrugia, L.J. PLATON, version 29-11-98; Windows implementation; University of Glasgow, 1998.
Hu, N.H.; Liu, Y.S. Acta Crystallogr., Sect. C, 1991, 47, 2324.
Khan, M.A.; Tuck, D.G. Acta Crystallogr., Sect. C, 1984, 40, 60.
(a) Janiak, C.; Deblon, S.; Wu, H.-P.; Kolm, M.J.; Klüfers, P.; Piotrowski, H.; Mayer, P. Eur. J. Inorg. Chem. 1999, 1507; (b) Janiak, C.; Temizdemir, S.; Scharmann, T.G.; Schmalstieg, A.; Demtschuk, J. Z. Anorg. Allg. Chem. 2000, 626 2053; (c) Burrows, A.D.; Harrington, R.W.; Mahon, M.F.; Price, C.E. J. Chem. Soc., Dalton Trans. 2000, 3845; (d) Plater, J.M.; Foreman, M.R. St. J.; Gelbrich, T.; Coles, S.J.; Hursthouse, M.B. J. Chem. Soc., Dalton Trans. 2000, 3065; (e) Janiak, C.; Scharmann, T.G.; Hemling, H.; Lentz, D.; Pickardt, J. Chem. Ber. 1995, 128 235; (f ) Janiak, C.; Scharmann, T.G.; Günther, W.; Girgsdies, F.; Hemling, H.; Hinrichs, W.; Lentz, D. Chem. Eur. J. 1995, 1 637.
(a) Janiak, C. J. Chem. Soc., Dalton Trans. 2000, 3885; and references therein; (b) Blake, A.J.; Champness, N.R.; Cooke, P.A.; Nicolson, J.E.B.;Wilson, C. J. Chem. Soc., Dalton Trans. 2000, 3811; (c) Nakash, M.; Clyde-Watson, Z.; Feeder, N.; Teat, S.J.; Sanders, J.K.M. Chem. Eur. J. 2000, 6 2112.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhang, C., Janiak, C. Six-coordinated zinc complexes: [Zn(H2O)4(phen)] (NO3)2·H2O and [ZnNO3(H2O)(bipy)(Him)]NO3 (phen = 1,10-phenanthroline, bipy = 2,2′-bipyridine, and Him = imidazole). Journal of Chemical Crystallography 31, 29–35 (2001). https://doi.org/10.1023/A:1013774502147
Issue Date:
DOI: https://doi.org/10.1023/A:1013774502147