Abstract
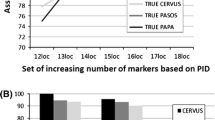
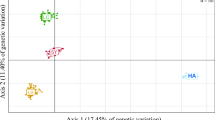
Hatchery broodstocks used for genetic conservation or aquaculture may represent their ancestral gene pools rather poorly. This is especially likely when the fish that found a broodstock are close relatives of each other. We re-analysed microsatellite data from a breeding experiment on red sea bream to demonstrate how lost genetic variation might be recovered when gene frequencies have been distorted by consanguineous founders in a hatchery. A minimal-kinship criterion based on a relatedness estimator was used to select subsets of breeders which represented the maximum number of founder lineages (i.e., carried the fewest identical copies of ancestral genes). UPGMA clustering of Nei's genetic distances grouped these selected subsets with the parental gene pool, rather than with the entire, highly ‘drifted’ offspring generation. The selected subsets also captured much of the expected heterozygosity and allelic diversity of the parental gene pool. Independent pedigree data on the same fish showed that the selected subsets had more contributing parents and more founder equivalents than random subsets of the same size. The estimated mean coancestry was lower in the selected subsets, meaning that inbreeding in subsequent generations would be lower if they were used as breeders. The procedure appears suitable for reducing the genetic distortion due to consanguineous and over-represented founders of a hatchery gene pool.
Similar content being viewed by others
References
Ballou, J.D., 1983. Calculating inbreeding coefficients from pedigrees, pp. 509–520 in Genetics and Conservation, edited by C.M. Schoenwald-Cox, S.M. Chambers, F. MacBryde & L. Thomas. Benjamin-Cummings, Menlo Park, CA.
Ballou, J.D. & R.C. Lacy, 1995. Identifying genetically important individuals for management of genetic variation in pedigreed populations, pp. 76–111 in Population Management for Survival and Recovery: Analytical Methods and Strategies in Small Populations, edited by J. Ballou, M. Gilpin & T.J. Foose. Columbia University Press, New York.
Bijma, P. & J.A. Woolliams, 2000. Prediction of rates of inbreeding in populations selected on best linear unbiased prediction of breeding value. Genetics 156: 361–373.
Caballero, A., 1994. Developments in the prediction of effective population size. Heredity 73: 657–679.
Caballero, A. & M.A. Toro, 2000. Interrelations between effective population size and other pedigree tools for the management of conserved populations. Genet. Res. 75: 331–343.
Chesser, R.K., 1991a. Gene diversity and female phylopatry. Genetics 127: 437–447.
Chesser, R.K., 1991b. Influence of gene flow and breeding tactics on gene diversity within populations. Genetics 129: 573–583.
Cornuet, J.-M. & G. Luikart, 1996. Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144(4): 2001–2014.
Crow, J.F. & M. Kimura, 1970. An Introduction to Population Genetics Theory. Harper & Row, New York, 591 p.
Doyle, R.W., 1999. Managing broodstock for on-farm genetic improvement: principles and examples, pp. 155–162 in Proceedings Simposio Centroamericano de Acuacultura, edited by B.W. Green, H.C. Clifford, M. McNamara & G.M. Moctezuma. World Aquaculture Society (C.R.S.P.), San Pedro Sula, Honduras.
Doyle, R.W. & C.M. Herbinger, 1994. The use of DNA fingerprinting for high-intensity, within-family selection in fish breeding, pp. 364–371 in Proceedings 5th World Congress, Genetics Applied to Livestock Production Guelph, Vol. 19, Ontario, Canada.
Doyle, R.W. & C.M. Herbinger, 1995. Broodstock improvement strategies based on DNA fingerprinting: examples and cost-bene-fit analysis. Aquaculture 137: 283.
Ebenhard, T., 1995. Conservation breeding as a tool for saving animal species from extinction. Trends Ecol. Genet. 11(11): 438–443.
Falconer, D.S. & T.F.C. Mackay, 1996. Introduction to Quantitative Genetics. Longman, Harlow (UK), 4th edn, 464 p.
Fiumera, A.C., P.G. Parker & P.A. Fuerst, 2000. Effective population size and maintenance of genetic diversity in captivebred populations of a Lake Victoria cichlid. Conserv. Biol. 14: 886–892.
Frankham, R., 1995. Conservation genetics. Ann. Rev. Genet. 29: 305–327.
Hansen, M.M., E.E. Nielsen, D.E. Ruzzante, C. Bouza & K.-L.D. Mensberg, 2000. Genetic monitoring of supportive breeding in brown trout (Salmo trutta L.), using microsatellite DNA markers. Can. J. Fisher. Aquat. Sci. 57: 2130–2139.
Hansen, M.M., D.E. Ruzzante, E.E. Nielsen & K-L.D. Mensberg, 2001. Brown trout (Salmo trutta) stocking impact assessment using microsatellite DNA markers. Ecol. Appl. 11: 148–160.
Hedrick, P.W., T.E. Dowling, W.L. Minckley, C.A. Tibbets, B.D. Demarais & P.C. Marsh, 2000a. Establishing a captive broodstock for the endangered Bonytail chub (Gila elegans). J. Heredity 91: 35–39.
Hedrick, P.W., D. Hedgecock, S. Hamelberg & S.J. Croci, 2000b. The impact of supplementation in winter-run chinook salmon on effective population size. J. Heredity 91: 112–116.
Hedrick, P.W. & P.S. Miller, 1992. Conservation genetics: techniques and fundamentals. Ecol. Appl. 2(1): 30–46.
Hedrick, P.W., V.K. Rashbrook & D. Hedgecock, 2000. Effective population size of winter-run chinook salmon based on microsatellite analysis of returning spawners. Can. J. Fisher Aquat. Sci. 57: 2368–2373.
Hill, W.G., 1996. Sewall Wright' 'systems of mating'. Genetics 143: 1499–1506.
Hill, W.G., A. Caballero & L. Dempfle, 1996. Prediction of response to selection within families. Genet. Select. Evol. 28: 379–383.
Hindar, K., N. Ryman & F.M. Utter, 1991. Genetic effects of aquaculture on natural fish populations. Can. J. Fisher. Aquat. Sci. 48: 945–957.
Kinghorn, B. & S. Kinghorn, 2000. Pedigree viewer.
Lacy, R.C., 1989. Analysis of founder representation in pedigrees: founder equivalents and founder genome equivalents. Zoo Biol. 8: 111–124.
Lacy, R.C., J.D. Ballou, F. Princee, A. Starfield & E. Thompson, 1995. Pedigree analysis for population management, pp. 57–75 in Population Management for Survival and Recovery: Analytical Methods and Strategies in Small Populations, edited by J. Ballou, M. Gilpin & T.J. Foose. Columbia University Press, New York.
Lens, L., S. Van Dongen, P. Galbusera, T. Schenck, E. Matthysen & T. Van De Casteele, 2000. Developmental instability and inbreeding in natural bird populations exposed to different levels of habitat disturbance. J. Evol. Biol. 13(6): 889–896.
Lewis, P.O. & D. Zaykin, 2001. GDA (Genetic Data Analysis).
McAndrew, B.J., K.J. Rana & D.J. Penman, 1992. Conservation and preservation of genetic variation in aquatic organisms, pp. 298–336 in Recent Advances in Aquaculture IV, edited by J. F. Muir & R.J. Roberts. Blackwell.
Mehrabani-Yeganeh, H., J.P. Gibson & L.R. Schaeffer, 2000. Including coefficients of inbreeding in BLUP evaluation and its effect on response to selection. J. Anim. Breed. Genet. 117(3): 145–151.
Montgomery, M.E., J. Ballou, R.K. Nurthen, P.R. England, D.A. Briscoe & R. Frankham, 1997. Minimizing kinship in captive breeding programs. Zoo Biol. 16(5): 377–389.
Moritz, C., 1999. Conservation units and translocations: strategies for conserving evolutionary processes. Hereditas 130: 217–228.
Mosseau, T.A., K. Ritland & D.D. Heath, 1998. A novel method for estimating heritability using molecular markers. Heredity 80: 218–224.
Nei, M., 1988. Molecular Evolutionary Genetics. Columbia University Press, 512 p.
Nevo, E., 1978. Genetic variation in natural populations: patterns and theory. Theoret. Popul. Biol. 13: 121–177.
Nielsen, E.E., M.M. Hansen & V. Loeschke, 1999. Analysis of DNA from old scale samples: technical aspects, applications and perspectives for conservation. Hereditas 130: 265–276.
Nomura, T., 1999. Effective population size in supportive breeding. Conserv. Biol. 13(3): 670–672.
Pante, Ma.J.R., B. Gjerde & I. McMillan, 2001a. Effect of inbreeding on body weight at harvest in rainbow trout, Oncorhynchus mykiss. Aquaculture 192(2-4): 201–211.
Pante, Ma.J.R., B. Gjerde & I. McMillan, 2001b. Inbreeding levels in selected populations of rainbow trout, Oncorhynchus mykiss. Aquaculture 192(2-4): 213–224.
Perez-Enriquez, R., M. Takagi & N. Taniguchi, 1999. Genetic variability and pedigree tracing of a hatchery-reared stock of red sea bream (Pagrus major) used for stock enhancement, based on microsatellite DNA markers. Aquaculture 173: 143–423.
Pouyaud, L, E. Desmarais, A. Chenuil, J.F. Agnese & F. Bonhomme, 1999. Kin cohesiveness and possible inbreeding in the mouthbrooding tilapia Sarotherodon melanotheron (Pisces Cichlidae). Mol. Ecol. 8: 803–812.
Pray, L.A. & C.J. Goodknight, 1995. Genetic depression in inbreeding depression in the red flour beetle Tribolium cansaneum. Evolution 49: 176–188.
Primmer, C.R, T. Aho, J., Piironen, A. Estoup, J.M. Cornuet & E. Ranta, 1999. Microsatellite analysis of hatchery stocks and natural populations of Arctic charr, Salvelinus alpinus, from the Nordic region: implications for conservation. Hereditas 130: 277–289.
Queller, D.C. & K.F. Goodknight, 1989. Estimating relatedness using genetic markers. Evolution 43: 258–275.
Raymond, M. and F. Rousset, 1995. GENEPOP (V. 1.2) population genetics software for exact tests and ecumenicism. J. Heredity 86: 248–249.
Ritland, K., 1996a. Estimators for pairwise relatedness and individual inbreeding coefficients. Genet. Res. 67: 175–185.
Ritland, K., 1996b. A marker-based method for inferences about quantitative inheritance in natural populations. Evoution 50(3): 1062–1073.
Ritland, K., 2000. Marker-inferred relatedness as a tool for detecting heritability in nature. Mol. Ecol. 9(9): 1195–1204.
Rodriguez-Clark, K., 1999. Genetic theory and evidence supporting current practices in captive breeding for conservation, pp. 47–73 in Genetics and the Extinction of Species, edited by L.F. Landweber and A.P. Dobson. Princeton University Press, Princeton.
Ruzzante, D.E., M.M. Hansen & D. Meldrup, 2001. Individual and population level analysis of microsatellite variability in a geographically structured complex of resident and anadromous brown trout (Salmo trutta) subjected to stocking. Mol. Ecol. 10: 2107–2128.
Ryman, N., 1994. Supportive breeding and effective population size: differences between inbreeding and variance effective numbers. Conserv. Biol. 8: 888–890.
Ryman, N., P.E. Jorde & L. Laikre, 1995. Supportive breeding and variance effective population size. Conserv. Biol. 9(6): 1619–1628.
Ryman, N., P.E. Jorde & L. Laikre, 1999. Supportive breeding and inbreeding effective number: reply to Nomura. Conserv. Biol. 13: 673–676.
Ryman, N. & L. Laikre, 1991. Effects of supportive breeding on the genetically effective population size. Conserv. Biol. 5: 325–329.
Ryman, N., F. Utter & L. Laikre, 1995. Protection of intraspecific biodiversity of exploited fishes. Rev. Fish Biol. Fisher. 5: 417–446.
STATS 2000. Resampling Stats Inc., 612 N. Jackson Street, Arlington, VA.
SYSTAT 9 for Windows. SPSS Inc., 233 South Wacker Drive, Chicago, Ill.
Sugg, D.W. & R.K. Chesser, 1994. Effective population sizes with multiple paternity. Genetics 137(4): 11147–11155.
Sweigart, A., K. Karol, A. Jones & J.H. Willis, 1999. The distribution of individual inbreeding coefficients and pairwise relatedness in a population of Mimulus guttatus. Heredity 83: 625–632.
Taggart, J.B., I.S. McLaren, D.W. Hay, J.H. Webb & A.F. Youngson, 2001. Spawning success in Atlantic salmon (Salmo salar L.): a long-term DNA profiling-based study conducted in a natural stream. Mol. Ecol. 10(4): 1047–1060.
Taniguchi, N., K. Sumantadinata & S. Iyama, 1983. Genetic change in the first and second generations of hatchery stock of black seabream. Aquaculture 35: 309–320.
Tave, D., 1993. Genetics for Fish Hatchery Managers. Van Nostrand Reinhold, New York, 415 pp.
Tave, D., 1995. Selective breeding programmes for medium-sized fish farms. FAO Fisheries Technical Paper No. 352: FAO, Rome, 122 pp.
Templeton, A.R. & B. Read, 1994. Inbreeding: one word, several meanings, much confusion, pp. 91–105 in Conservation Genetics, edited by V. Loeschke, J. Tomiuk & S.K. Jain. Birkhauser Verlag, Basl.
Tessier, N., L. Bernatchez & J.M. Wright, 1997. Population structure and impact of supportive breeding inferred from mitochondrial and microsatellite DNA analyses in land-locked Atlantic salmon Salmo salar L. Mol. Ecol. 6: 735–760.
Tringali, M.D. & T.M. Bert, 1998. Risk to genetic effective population size should be an important consideration in fish stock-enhancement programs. Bull. Marine Sci. 62(2): 641–659.
Uraiwan, S. & R.W. Doyle, 1986. Replicate variance and the choice of selection procedure for tilapia (Oreochromis niloticus) stock improvement in Thailand. Aquaculture 48: 143–157.
Utter, F., 1998. Genetic problems of hatchery-reared progeny released into the wild and how to deal with them. Bull. Marine Sci. 62(2): 623–640.
Wang, J. & N. Ryman. 2001. Genetic effects of multiple generations of supportive breeding. Conserv. Biol. (in press).
Weir, B.S. & C.C. Cockerham, 1984. Estimating F-statistics for the analysis of population structure. Evolution 38: 1358–1370.
Wray, N.R. & R. Thompson, 1990. Predictions of rates of inbreeding in selected populations. Genet. Res. 55: 41–54.
Wright, S., 1969. Evolution and the Genetics of Populations. 4 Vols., Vol. 2, University of Chicago Press, 511 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doyle, R., Perez-Enriquez, R., Takagi, M. et al. Selective recovery of founder genetic diversity in aquacultural broodstocks and captive, endangered fish populations. Genetica 111, 291–304 (2001). https://doi.org/10.1023/A:1013772205330
Issue Date:
DOI: https://doi.org/10.1023/A:1013772205330