Abstract
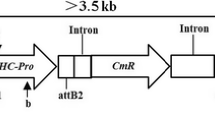
Development of transgenic disease resistance in soybeans, despite progress in other important crop plants, has advanced slowly. In this study, transgenic soybean plants resistant to soybean mosaic virus (SMV) were obtained by transforming with the coat protein gene and the 3′-UTR from SMV. Four insertion events were detected in a T0 plant obtained by using Agrobacterium tumefaciens-mediated transformation. Self-pollination of T0 progeny yielded four homozygous transgenic lines with a single insertion event or combinations of two insertion events in the T3 generation. A single coat protein gene transcript was detected in all four transgenic lines, and virus coat protein was detected in three transgenic lines. Two transgenic lines were highly resistant to the virus. These constitute the first example of stable genetically engineered disease resistance in soybean.
Similar content being viewed by others
References
Altenbach S.B., Pearson K.W., Meeker G., Staraci L.C. and Sun S.S.M. 1989. Enhancement of methionine content of seed proteins by expression of a chimeric gene encoding a methioninerich protein in transgenic plants. Plant Mol. Biol. 13: 513–522.
Barnett O.W. 1992. Potyvirus taxonomy. Arch. Virol. Supplem. 5: 435–450.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Brakke M.K. and van Pelt N. 1970. Properties of infectious ribonucleic acid from wheat streak mosaic virus. Virology 42: 699–706.
Bryant G.R., Hill J.H., Bailey T.B., Tachibana H., Durand D.P. and Benner H.I. 1982. Detection of soybean mosaic virus in seed by solid-phase radioimmunoassay. Plant Dis. 66: 693–695.
Buzzel R.I. and Tu J.C. 1984. Inheritance of soybean resistance to soybean mosaic virus. J. Hered. 75: 82.
Cervera M.T., Reichmann J.L., Martin M.T. and Garcia J.A. 1993. 3′-terminal sequence of the plum pox virus PS and 06 isolates: evidence for RNA recombination within the potyvirus group. J. Gen. Virol. 74: 329–334.
Cho E.K. and Goodman R.M. 1979. Strains of soybean mosaic virus: classification based on virulence in resistant soybean cultivars. Phytopathology 69: 467–470.
Cho E.K., Chung B.J. and Lee S.H. 1977. Studies on identification and classification of soybean virus disease in Korea. II. Etiology of a necrotic disease of Glycine max. Plant Dis. Rep. 61: 313–317.
Chomczynski P. and Mackey K. 1994. One-hour downward capillary blotting of RNA at neutral pH. Anal. Biochem. 221: 303–305.
Demski J.W. and Jellum M.D. 1975. Single and double virus infection of soybean: plant characteristics and chemical composition. Phytopathology 65: 1154–1156.
Dhingra K.L. and Chenulu V.V. 1980. Effect of soybean mosaic virus on yield and nodulation of soybean cv. Bragg. Ind. Phytopath. 33: 586–590.
Di R., Purcell V., Collins G.B. and Ghabrial S.A. 1996. Production of transgenic soybean lines expressing the bean pod mottle virus coat protein precursor gene. Plant Cell Rep. 15: 746–750.
Dougherty W.G. and Carrington J.C. 1988. Expression and function of potyviral gene products. Annu. Rev. Phytopath. 26: 123–143.
Eggenberger A.L., Stark D.M. and Beachy R.N. 1989. The nucleotide sequence of a soybean mosaic virus coat protein-coding region and its expression in Escherichia coli, Agrobacterium tumefaciens and tobacco callus. J. Gen. Virol. 70: 1853–1860.
El-Amrety A.A., El-Said H.M. and Salem D.E. 1985. Effect of soybean mosaic virus infection on quality of soybean seed. Agric. Res. Rev. 63: 155–164.
Fehr W.R. and Caviness C.E. 1977. Stages of soybean development. Iowa Agric. Home Econ. Exp. Stn. Spec. Rep. 80: 1–12.
Fitchen J.H. and Beachy R.N. 1993. Genetically engineered protection against viruses in transgenic plants. Annu. Rev. Microbiol. 47: 739–763.
Gal-On A., Antignus Y., Rosner A. and Raccah B. 1992. A zucchini yellow mosaic virus coat protein gene mutation restores aphid transmissibility but has no effect on multiplication. J. Gen.Virol. 73: 2183–2187.
Hari V. 1981. The RNA of tobacco etch virus: further characterization and detection of protein linked to RNA. Virology 112: 391–399.
Hill J.H. and Benner H.I. 1980. Properties of soybean mosaic virus and its isolated protein. Phytopath. Z. 97: 272–281.
Hill J.H. and Durand D.P 1986. Soybean mosaic virus. In: Bergmeyer H.U. (ed.), Methods of Enzymatic Analysis. VCH Verlagsgesellschaft, Weinheim, Germany, pp. 455–474.
Hill J.H., Lucas B.S., Benner H.I., Tachibana H., Hammond R.B. and Pedigo L.P. 1980. Factors associated with the epidemiology of soybean mosaic virus in Iowa. Phytopathology 70: 536–540.
Hill J.H., Benner H.I., Permar T.A., Bailey T.B., Andrews R.E., Durand D.P. and van Deusen R.A. 1989. Differentiation of soybean mosaic virus isolates by one-dimensional trypsin peptide maps immunoblotted with monoclonal antibodies. Phytopathology 79: 1261–1265.
Irwin M.E. and Goodman R.M. 1981. Ecology and control of soybean mosaic virus. In: Maramorosch K. and Harris K.F. (eds.), Plant Diseases and Their Vectors: Ecology and Epidemiology. Academic Press, New York, pp. 181–220.
Jayaram Ch., Hill J.H. and Miller W.A. 1992. Complete nucleotide sequences of two soybean mosaic virus strains differentiated by response of soybean containing the Rsv resistance gene. J. Gen. Virol. 73: 2067–2077.
Laemmli U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Lain S., Riechmann J.L., Mendez E. and Garcia J.A. 1988. Nucleotide sequence of the 3′ terminal region of plum pox potyvirus RNA. Virus Res. 10: 325–342.
Lim S.M. 1985. Resistance to soybean mosaic virus in soybeans. Phytopathology 75: 199–201.
Logemann J., Schell J. and Willmitzer L. 1987. Improved method for the isolation of RNA from plant tissues. Anal. Biochem. 163: 16–20.
Lomonosoff G.P. 1995. Pathogen-derived resistance to plant viruses. Annu. Rev. Phytopath. 33: 323–343.
Nutter F.W. Jr., Schultz P.M. and Hill J.H. 1998. Quantification of within-field spread of soybean mosaic virus in soybean using strain-specific monoclonal antibodies. Phytopathology 88: 895–901.
Padgette S.R., Kolacz K.H., Delannay X., Re D.B., LaValle B.J., Richolz D.A., Peschke V.M., Nida D.L., Taylor N.B. and Kishore G.M. 1995. Development, identification, and characterization of a glyphosate-tolerant soybean line. Crop Sci. 35: 1451–1461.
Powell-Abel P., Nelson R.S., De B., Hoffmann N., Rogers S.G., Fraley R.T. and Beachy R.N. 1986. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232: 738–743.
Revers F., Le Gall O., Candresse T., Le Romancer M. and Dunez J. 1996. Frequent occurrence of recombinant potyvirus isolates. J. Gen. Virol. 77: 1953–1965.
Ross J.P. 1969. Effect of time and sequence of inoculation of soybean with soybean mosaic and bean pod mottle viruses on yields and seed characters. Phytopathology 59: 1404–1408.
Saghai-Maroof M.A., Soliman K.M., Jorgensen R.A. and Allard R.W. 1984. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc. Natl. Acad. Sci. USA. 81: 8014–8018.
Sanford J.D. and Johnson S.A. 1985. The concept of parasitederived resistance. J. Theor. Biol. 115: 395–405.
Shah D.M., Rommens C.M.T. and Beachy R.N. 1995. Resistance to diseases and insects in transgenic plants: progress and applications to agriculture. Trends Biotechnol. 13: 362–368.
Townsend J.A. and Thomas L.A. 1996. Method of Agrobacteriummediated transformation of cultured soybean cells. U.S. Patent 5563055.
Waterhouse P.M., Smith N.A. and Wang M.-B. 1999. Virus resistance and gene silencing: killing the messenger. Trends Plant Sci. 4: 452–457.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Eggenberger, A.L., Nutter, F.W. et al. Pathogen-derived transgenic resistance to soybean mosaic virus in soybean. Molecular Breeding 8, 119–127 (2001). https://doi.org/10.1023/A:1013358200107
Issue Date:
DOI: https://doi.org/10.1023/A:1013358200107