Abstract
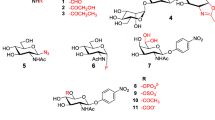
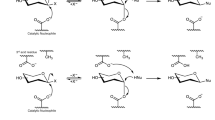
The ability of four exoglycosidases (β-galactosidase, β-glucosidase, α-glucosidase and invertase) from the termite Macrotermes subhyalinus to catalyse tranglycosylation reactions was tested using lactose, cellobiose, maltose and sucrose as glycosyl donors and 2-phenylethanol as glycosyl acceptor. The experimental conditions were optimized in relation to the time course of the reaction, pH and concentrations of glycosyl donor and acceptor. Whereas the hydrolytic activity was largely predominant over the transferase activity with β-galactosidase and β-glucosidase, the transglycosylation activity represented 68% with α-glucosidase. In addition, as demonstrated by the transglycosylation product formed, the hydrolysis of sucrose was catalysed by α-glucosidase and not by invertase. On the basis of this work, α-glucosidase from M. subhyalinus appears to be a valuable tool for the preparation of neoglycoconjugates.
Similar content being viewed by others
References
Becker KC,Kuhl P (1999) Synthesis of O-β-galactopyranosyl-L-serine derivatives using β-galactosidase in aqueous-organic reaction systems J. Carbohydr. Chem. 18: 121-129.
Chang ST,Parker KN,Bauer MW,Kelly RM (2001) β-Glucosidase from Pyrococcus furiosus. Meth. Enzymol. 330: 260-269.
Cote GL,Tao BY (1990) Oligosaccharide synthesis by enzymatic transglycosylation. Glycoconjugate J. 7: 145-162.
Crout DHG,Vic G (1998) Glycosidases and glycosyl transferases in glycoside and oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2: 98-111.
Dion M,Fourage L,Hallet JN,Colas B (1999) Cloning and expression of a β-glycosidase gene from Thermus thermophilus. Sequence and biochemical characterization of the encoded enzyme. Glycoconjugate J. 16: 27-37.
Finch P,Yoon JH (1997) The effects of organic solvents on the synthesis of galactose disaccharides using β-galactosidases. Carbohydr. Res. 303: 339-345.
Fortun Y,Colas B (1991) Lithium chloride effect on phenylethyl-β-D-galactoside synthesis by Aspergillus oryzae β-D-galactosidase in the presence of high lactose concentration. Biotechnol. Lett. 13: 863-866.
Fourage L,Helbert M,Nicolet P,Colas B (1999) Temperature dependence of the ultraviolet-visible spectra of ionized and un-ionized forms of nitrophenol: consequence for the determination of enzymatic activities using nitrophenyl derivatives-A warning. Anal. Biochem. 270: 184-185.
Gijsen HJM,Qiao L,Fitz W,Wong CH (1996) Recent advances in the chemoenzymatic synthesis of carbohydrates and carbohydrate mimetics. Chem. Rev. 96: 443-473.
Hoefsloot LH,Hoogeveen-Westerveld M,Kroos MA,van Beeumen J,Reuser AJJ,Oostra BA (1988) Primary structure and processing of lysosomal β-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 7: 1697-1704.
Ichikawa Y,Look GC,Wong CH (1992) Enzyme-catalyzed oligosaccharide synthesis. Anal. Biochem. 202: 215-238.
Kunst A,Draeger B,Ziegenhorn J (1984) Colorimetric methods with glucose oxidase and peroxidase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis, Vol. 6. Weinheim: Verlag Chemie, pp. 178-185.
Leparoux S,Fortun Y,Colas B (1994) Synthesis of β-galactosyl-(hydroxy amino acid) derivatives using β-galactosidase activity of Achatina achatina digestive juice. Biotechnol. Lett. 16: 677-682.
Leparoux S,Padrines M,Fortun Y,Colas B (1996) O-glycosylation of dipeptides using β-galactosidase activity of Achatina achatina digestive juice. Biotechnol. Lett. 18: 135-138.
Leparoux S,Padrines M,Placier G,Colas B (1997) Characterization of a strictly specific acid β-galactosidase from Achatina achatina. Biochim. Biophys. Acta 1336: 522-532.
Matoub M (1993) La symbiose termite-champignon chez Macrotermes bellicosus (Termitidae Macrotermitinae) Rôle des enzymes acquises dans la xylanolyse. Thesis, University Paris XII, Valde-Marne.
Nakao M,Nakayama T,Harada M,Kakudo A,Ikemoto H,Kobayashi S,Shibano Y (1994) Purification and characterization of a Bacillus sp. SAM1606 thermostable β-glucosidase with transglucosylation activity. Appl. Microbiol. Biotechnol. 44: 337-343.
Nilsson KGI (1988) Enzymatic synthesis of oligosaccharides. Trends Biotechnol. 6: 256-264.
Rouland C (1986) Contribution à l'étude des osidases digestives de plusieurs espèces de termites africains. Purification et caractérisation des cellulases et xylanases de Macrotermes mülleri (Termitidae, Macrotermitinae et de son champignon symbiotique. Thesis, University Paris Val-de-Marne.
Rouland C,Brauman A,Keleke S,Labat M,Mora P,Renoux J (1990) Endosymbiosis and exosymbiosis in the fungus-growing termites. In: Lesel R, ed. Microbiology in Poecilotherms. Amsterdam: Elsevier Science, pp. 79-82.
Singh S,Scigelova M,Vic G,Crout DHG (1996) Glycosidasecatalysed oligosaccharide synthesis of di-, tri-and tetrasaccharides using the N-acetylhexosaminidase from Aspergillus oryzae and the β-galactosidase from Bacillus circulans. J. Chem. Soc. Perkin Trans I: 1921-1926.
Toone EJ,Simon ES,Bednarski MD,Whitesides GM (1989) Enzyme-catalyzed synthesis of carbohydrates. Tetrahedron 45: 5365-5422.
Veivers PC,Mühlemann R,Slaytor M,Leuthold RH,Bignell DE (1991) Digestion, diet and polyethism in two fungus-growing termites: Macrotermes subhyalinus Rambur and M. michaelseni Sjøstedt. J. Insect. Physiol. 37: 675-682.
Vetere A,Galateo C,Paoletti S (1997) All-aqueous, regiospecific transglycosylation synthesis of 3-O-β-L-fucopyranosyl-2-acetamido-2-deoxy-D-glucopyran ose, a building block for the synthesis of branched oligosaccharides. Biochem. Biophys. Res. Commun. 234: 358-361.
Vulfson EN,Patel R,Beecher JE,Andrews AT,Law BA (1990) Glycosidases in organic solvents: I. Alkyl-β-glucoside synthesis in a water-organic two-phase system. Enzyme Microbiol. Tech. 12: 950-954.
Wong CH,Halcomb RL,Ichikawa Y,Kajimoto T (1995) Enzymes in organic synthesis: application to the problems of carbohydrate recognition (part 2). Angew. Chem. Int. Ed. Engl. 34: 521-546.
Yamamoto I,Muto N,Nagata E,Nakamura T,Suzuki Y (1990) Formation of a stable L-ascorbic acid β-glucoside by mammalian β-glucosidase-catalyzed transglucosylation. Biochim. Biophys. Acta 1035: 44-50.
Yoon JH,Ajisaka K (1996) The synthesis of galactopyranosyl derivatives with β-galactosidases of different origins. Carbohydr. Res. 292: 153-163.
Zeng X,Yoshino R,Murata T,Ajisaka K,Usui T (2000) Regioselective synthesis of p-nitrophenyl glycosides of β-Dgalactopyranosyl-disaccharides by transglycosylation with β-Dgalactosidases. Carbohydr. Res. 325: 120-131.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kouamé, L.P., Niamké, S., Diopoh, J. et al. Transglycosylation reactions by exoglycosidases from the termite Macrotermes subhyalinus. Biotechnology Letters 23, 1575–1581 (2001). https://doi.org/10.1023/A:1011969310742
Issue Date:
DOI: https://doi.org/10.1023/A:1011969310742