Abstract
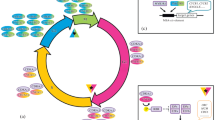
The aim was to review knowledge about the interface betweenplant growth regulators and molecular checkpoints of the cell cycle. Atwhat level of biochemical regulation of the cell cycle do plant growthregulators interface? Are there different levels of interfacingdependent on the plant growth regulator involved? As a preamble totackling these questions, we overview the eukaryotic cell cycle withparticular emphasis on checkpoints that regulate the transition fromG0-G1-S-phase and G2-M. Cytokinins feature strongly as activators ofcell division in plants both in vivo and in vitro.Recent research has shown that zeatin treatment led to the up-regulationof CycD3 in Arabidopsis. This is a D-type cyclin showing stronghomology with vertebrate D cyclins which themselves are up-regulated byextracellular growth factors. Benzyladenine treatment can also shortenthe duration of S-phase through recruitment of latent origins of DNAreplication. Kinetin is involved in the phosphoregulation of the G2-Mcheckpoint; the major cyclin-dependent kinase (Cdk) at this checkpointhas recently been shown to be dephosphorylated as a result of cytokinintreatment, an effect which can also be mimicked by the fission yeastCdc25 phosphatase. Hence, a picture emerges of a cytokinin-inducedcontinuum of cell cycle activation through the up-regulation of a plantD-type cyclin at the G1 checkpoint and the phosphoregulation of the Cdkat the G2/M checkpoint. During S-phase, we argue for a link betweencytokinins and the proteins associated with replication origins.Gibberellic acid (GA) treatment induces internode elongation. Indeepwater rice, this response is mediated, at least partly, by aGA-induced up-regulation of a cyclin-Cdk at the G2-M checkpoint. Recentevidence has also linked abscisic acid to a cyclin-dependent kinaseinhibitor. These, so-called CKIs are negative regulators of Cdks whichfits with ABA's general role in growth inhibition; we await news ofethylene interactions. We highlight two instances of plant growthregulator-cell cycle interfacing during development, arguing for aninvolvement in microtubule orientation as a prerequisite to leafinitiation, and suggest a link between IAA and the activation of celldivisions in the pericycle required for lateral root initiation. A newD-type cyclin, recently discovered in Arabidopsis, may have akey role in this process. Finally, a model is presented which features ageneralised cyclin-Cdk checkpoint exhibiting various interfaces with theplant growth regulators.
Similar content being viewed by others
References
Ach RA, Durfee T, Miller AB, Taranto P, Hanley-Bowdoin L, Zambryski PC and Gruissem W (1997) RRB1 and RRB2 encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein. Mol Cell Biol 17: 5077–5086
Bayliss M (1985) Control of cell division in cultured cells. In: Bryant JA and Francis D (eds) The Cell Division Cycle in Plants. Cambridge: Cambridge University Press, pp 157–178
Berridge MJ (1995) Calcium signalling and cell proliferation. Bioessays 17: 491–500
Bielinsky AK and Gerbi SA (1998) Discrete start sites for DNA synthesis in the yeast ARS1 origin. Science 279: 95–98
Blakely LM, Durham M, Evans TA and Blakely RM (1982) Experimental studies on lateral root formation in radish seedlings. I General methods, developmental stages, and spontaneous formation of laterals. Bot Gaz 143: 341–352
Bleeker AB, Schuette JL and Kende H (1986) Anatomical analysis of growth and developmental patterns of deepwater rice. Planta 169: 490–497
Bryant JA (1998) Biochemical aspects of S-phase regulation in the plant cell cycle. In: Bryant JA and Chiatante D (eds), Plant Cell Proliferation and its Regulation in Growth and Development. Chichester, NY: J. Wiley, pp 31–44
Celenza JL, Grisafi PL and Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142
Conklin DS, Galaktionov K and Beach D (1995) 14-3-3 proteins associate with cdc25 phosphatases. Proc Natl Acad Sci USA 92: 7892–7896
Cooke R and Meyer Y(1981) Hormonal control of tobacco protoplast nucleic acid metabolism during in vitro culture. Planta 152: 1–7
Crowell DN and Salaz MS(1992) Inhibition of growth of cultured tobacco cells at low concentrations of lovostatin is reversed by cytokinin. Plant Physiol 100: 2090–2095
Dahl M, Meskiene I, Bögre L, Ha DTC, Swoboda I, Hubmann R, Hirt H and Heberle-Bors E (1995) The D-type alfalfa cyclin gene cycMs4 complements G1 cyclin-deficient yeast and is induced in the G1 phase of the cell cycle. Plant Cell 7: 1847–1857
Das, NK, Patau K and Skoog F (1956) Initiation of mitosis and cell division by kinetin and indoleacetic acid in excised tobacco pith tissue. Physiol Plant 9: 640–651
Daykin A, Scott IM, Causton DR and Francis D (1997) Gibberellin does not accelerate rates of cell division in the dwarf pea shoot apical meristem. J Exp Bot 48: 1147–1150
Daykin A, Scott IM, Francis D and Causton DR (1997) Effects of gibberellin on the cellular dynamics of dwarf pea internode development. Planta 207: 235–240
De Veylder L, Almedia Engler J de, Burssens S, Manevski A, Lescure B, Van Montague M, Engler G and Inze D (1999) A new D-type cyclin of Arabidopsis thaliana expressed during lateral root primordia formation. Planta 208: 453–462
Donovan S, Harwood J, Drury LS and Diffley JF (1997) Cdc6p-dependent loading of Mcm proteins on prereplicative chromatin in budding yeast. Proc Natl Acad Sci USA 94: 5611–5616
Doonan JH (1998) Cell division during floral morphogenesis in Antirrhinum majus. In: Francis D, Dudits D and Inzé D. (eds) Plant Cell Division. London: Portland Press, pp 207–222
Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A and Winberg RA (1993) Physical interaction of the retinoblastoma protein with human D cyclins. Cell 73: 499–511
Durdan SF, Herbert RJ and Francis D (1998) Activation of latent origins of DNA replication in florally determined shoot meristems of long-day and short-day plants: Silene coeli-rosa and Pharbitis nil. Planta 207: 235–240
Dutta A and Bell SP (1997) Initiation of DNA replication in eukaryotic cells. Ann Rev Cell Dev Biol 13: 293–332
Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274: 1664–1672
Evans LS and Van't Hof J (1973) Cell arrest in G2 in root meristems. Exp Cell Res 82: 471–473
Evans LS and Van't Hof J (1974) Promotion of cell arrest in root and shoot meristems in Pisum by factor from the cotyledons. Exp Cell Res 87: 259–264
Fesquet D, Labbe J-C, Derancourt J, Capony J-C, Galsa S, Girand F, Lorca T, Shuttleworth J, Doree M and Cavadore JC (1993) The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J 12: 3111–3121
Fisher RP and Morgan DO (1994) A novel cyclin associates with MO15/CDK7 to form the CDK-activating kinase. Cell 78: 713–724
Fosket DE (1977) Regulation of the cell cycle by cytokinin. In: Rost TL and Gifford EM(eds) Mechanism and Control of Cell Division. Stroudsberg Pennsylvania: Dowden, Hutchinson and Ross, pp 62–91
Francis D (1998) Cell size and organ development in higher plants. In: Francis D, Dudits D and Inze D (eds) Plant Cell Division. Colchester: Portland Press, pp 187–206
Francis D and Lyndon RF (1985) The control of the cell cycle in relation to floral induction. In: Bryant JA and Francis D (eds) The Cell Division Cycle in Plants. Cambridge: Cambridge University Press, pp 199–215
Gonthier R, Jacqmard A and Bernier G (1987) Changes in cell cycle duration and growth fraction in the shoot meristems of Sinapis during floral transition. Planta 170: 55–59
Green PB (1994) Connecting gene and hormone action to form, pattern and organogenesis: biophysical transduction. J Exp Bot 45 (special issue): 1775–1788
Hartwell LH and Weinert TA (1989) Checkpoints: controls that ensure the order of cell cycle events. Science 246: 629–634
Hemerly AS, Ferreira P, de Almeida Engler J, Van Montague M, Engler G and Inze D (1993) cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell 5: 1711–1723
Houssa C, Jacqmard A and Bernier G (1990) Activation of replicon origins as possible targets for cytokinins in shoot meristems of Sinapis. Planta 181: 324–326
Huntley R, S, Freeman D, Lavender P, de Jager S, Greenwood J, Makkerh J, Walker E, Jackman M, Xie Q, Bannister AJ, Kouzarides T, Gutiérrez C, Doonan JH and Murray JAH (1998) The maize retinoblastoma protein homologue ZmRb-1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin D (CycD) proteins. Plant Mol Biol 37: 155–169
Ivanova M and Rost TL (1998) Cytokinins and the plant cell cycle: problems and pitfalls of proving their function. In: Bryant JA and Chiatante D (eds) Plant Cell Proliferation and its Regulation in Growth and Development. Chichester, NY: John Wiley and Sons, pp 45–57
Jackson AL, Pahl PM, Harrison K, Rosamond J and Sclafani RA (1993) Cell cycle regulation of the yeast Cdc7 protein kinase by association with the Dbf4 protein. Mol Cell Bio 13: 2899–2908
Jacobs TW (1997) Why do plant cells divide? Plant Cell 9: 1021–1029
Jacqmard A and Houssa C (1988) DNA fiber replication during a morphogenetic switch in the shoot meristematic cells of a higher plant. Exp Cell Res 179: 454–461
Jacqmard A, Houssa C and Bernier G (1994) Regulation of the cell cycle by cytokinins. In: Mok DWS and Mok MC (eds), Cytokinins, Chemistry, Activity and Function. Boca Raton Florida: CRC Press, pp 197–215
Jacqmard A, Houssa C and Bernier G (1995) Abscisic acid antagonizes the effect of cytokinin on DNA-replication origins. J Exp Bot 46: 663–666
Jacqmard A, Houssa C and Bernier G (1998) Control of the cell division cycle changes in the shoot meristem of Sinapis alba during the transition to flowering. In: Bryant JA, Chiatante D (Eds) Plant Cell Proliferation and its Regulation in Growth and Development. London: John Wiley, pp 67–78
Jiang W and Hunter T (1997) Identification and characterization of a human protein kinase related to budding yeast Cdc7p. Proc Natl Acad Sci USA 94: 14320–14325
Joanneau JP and Tandeau de Marsac N (1973) Stepwise effects of cytokinin activity upon tobacco cell division. Exp Cell Res 77: 167–174
Krek W and Nigg EA (1991) Differential phosphorylation of vertebrate p34cdc2 at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J 10: 305–316
Kumagai A, Yakowec PS and Dunphy WG (1998) 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell 9: 345–354
Laureys F, Dewitte W, Witters E, Van Montague M, Inze D and Van Onckelen H (1998) Zeatin is indispensable for the G2-M transition in tobacco BY-2 cells. FEBS Lett 426: 29–32
Leatherwood J (1998) Emerging mechanisms of eukaryotic DNA replication initiation. Cur Op Cell Biol 10: 742–748
Levine AJ (1997) p53, the cellular gatekeeper for growth and division. Cell 88: 323–331
Lundgren K, Walworth N, Booher R, Dembski M, Kirschner Mand Beach D (1991) mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 54: 1111–1122
Lyndon RF (1977) Interacting processes in development at the shoot apex. Symp SEB 31: 221–250
Lyndon RF (1979) Rates of growth and primordial initiation during flower development in Silene at different temperatures. Ann Bot 43: 539–551
Masuda Y (1990) Auxin-induced cell elongation and cell wall changes. Bot Mag 103: 345–370
Mazzuca S, Bitonti MB, Pranno S and Innocenti AM (1997) Nuclear metabolic changes in root meristem of Lactuca sativa induced by trigonelline. Cytobios 89: 39–50
Métraux J-P and Kende H (1983) The role of ethylene in the growth response of submerged deep water rice. Plant Physiol 72: 441–446
Miller CO, Skoog F, Von Slatzer MH and Strong FM (1955) Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc. 77: 1392
Mitchell EK and Davies PJ (1975) Evidence for three different systems of movement of indoleacetic acid in intact roots of Phaseolus coccineus. Physiol Plant 33: 290–294
Mironov V, DeVeylder L, Van Montague M and Inze D (1999) Cyclin-dependent kinases and cell division in plants – the nexus. Plant Cell 11: 509–521
Murray JAH, Freeman D, Greenwood J, Huntley R, Makkerh J, Riou-Khamlichi C, Sorrell DA, Cockcroft C, Carmichael JP, Soni R and Shah ZH (1998) Plant D cyclins and retinoblastoma protein homologues. In: Francis D, Dudits D and Inzé D (eds) Plant Cell Division. London: Portland Press, pp 99–127
Norbury C and Nurse P (1992) Animal cell cycles and their control. Ann Rev Biochem 61: 441–470
Nougarède A, Silveira CE and Rondet P (1996) In nature dormant buds and in vitro dormant-like buds of Fraxinus excelsior L. Protoplasma 190: 16–24
Nurse P (1990) Universal control mechanism regulating onset of M-phase. Nature 344: 503–508
Paulovich AG, Toczyski DP and Hartwell LH (1997) When checkpoints fail. Cell 88: 315–321
Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS and Piwcnica-Worms H (1997) Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277: 1501–1505
Ramírez-Parra E, Xie Q, Boniotti MB and Gutierrez C (1999) The cloning of plant E2F, a retinoblastoma-binding protein, reveals unique and conserved features with animal G1/S regulators. Nucleic Acids Res 27: 3527–3533
Redig P, Shaul O, Inze D, Van Montague M and Van Onckelen H (1996) Levels of endogenous cytokinins, indole-3-acetic acid and abscisic acid during the cell cycle of synchronised tobacco BY-2 cells. FEBS Lett 391: 175–180
Renaudin J-P, Doonan JH, Freeman D, Hashimoto J, Hirt H, Inzé D, Jacobs T, Kouchi H, Rouzé P, Sauter M, Savouré A, Sorrell DA, Sundaresan V and Murray JAH (1996) Plant cyclins: a unified nomenclature for plant A-, B-and D-type cyclins based on sequence organisation. Plant Mol Biol 32: 1003–1018
Rhind P and Russell P (1998) Mitotic DNA damage and replication checkpoints in yeast. Curr Op Cell Biol 10: 749–758
Riou-Khamlichi C, Huntley R, Jacqmard A and Murray JAH (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544
Russell P and Nurse P (1986) cdc25 + functions as an inducer in the mitotic control of fission yeast. Cell 45: 145–153
Russell P and Nurse P (1987a) Negative regulation of mitosis by wee1 +, a gene encoding a protein kinase homologue. Cell 49: 559–567
Russell P and Nurse P (1987b) The mitotic inducer nim1 + functions in a regulatory network of protein homologues controlling the initiation of mitosis. Cell 49: 569–576
Sauter M and Kende H (1992) Gibberellin-induced growth and regulation of the cell division cycle in deepwater rice. Planta 188: 362–368
Sauter M, Mekhedov SL and Kende H (1995) Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant Journal 7: 623–632
Schultze-Galmen U, Brandsen J, Jones HD, Morgan DO, Meijer L, Vesely J and Kim SH (1995) Multiple-modes of ligand recognition – crystal-structures of cyclin-dependent protein-kinase-2 in complex with ATP and 2 inhibitors, olomoucine and isopentenyladenine. Proteins-structure function and genetics 22: 378–391
Scott TK (1972) Auxins and roots. Ann Rev Plant Physiol 23: 235–258
Sekine M, Ito M, Uemukai K, Maeda Y, Nakagami H and Shinmyo A (1999) Isolation and characterisation of the E2Flike gene in plants. FEBS Lett 460: 117–122
Sherr CJ (1993) Mammalian G1 cyclins. Cell 73: 1059–1065
Sherr CJ (1996) Cancer cell cycles. Science 274: 1672–1677
Shibaoka H (1994) Plant hormone-induced changes in the orientation of cortical microtubules: Alterations in the crosslinking between microtubules and the plasma membrane. Ann Rev Plant Physiol Plant Mol Biol 45: 527–544
Shuttleworth J, Godfrey R and Colman A (1990) p40MO15, a cdc2-related protein kinase involved in negative regulation of meiotic maturation of Xenopus oocytes. EMBO J 9: 3233–3240
Snow M and Snow R (1931) Experiments on phyllotaxis I. The effects of isolating a primordium. Phil Trans Roy Soc Lond B 221: 1–43
Snow M and Snow R (1933) Experiments on phyllotaxis II. The effect of displacing a primordium. Phil Trans Roy Soc Lond B 222: 353–400
Snow M and Snow R (1937) Auxin and leaf formation. New Phytol 36: 1–18
Soni R, Carmichael JP, Shah ZH and Murray JAH (1995) A family of cyclin D homologs from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell 7: 85–103
Sorrell DA (1999) CycD cyclins and cyclin-dependent kinases in tobacco BY-2 cells. PhD thesis, Cambridge University, UK
Sorrell DA, Combettes B, Chaubet-Gigot N, Gigot C and Murray JAH (1999a) Distinct cyclin D genes show mitotic accumulation or constant levels of transcripts in tobacco Bright Yellow-2 cells. Plant Physiol 119: 343–351
Sorrell DA, Riou-Khamlichi C, Chaubet-Gigot N, Combettes B, Gigot C and Murray JAH (1999b) Plant D-type (CycD) cyclins and the regulation of the plant cell cycle. In: Altmann, A, Ziv M and Izhar S (eds) Plant Biotechnology and in vitro Biology in the 21st Century. Dordrecht: Kluwer Academic Publishers, pp 441–444
Springer P, McCombie RW, Sundaresan V and Martienssen RA (19995) Gene trap tagging of prolifera, an essential mcm2-3-5-like gene in Arabidopsis. Science 268: 877–880
Tanaka T, Knapp D and Nasmyth K (1997) Loading of an Mcm protein onto DNA replication origins is regulated by Cdc6p and CDKs. Cell 90: 649–660
Taylor JH (1963) DNA synthesis in relation to chromosome reproduction and the reunion of breaks. J Cell Comp Physiol 62: 73–80
Torrey JG (1965) Cytological evidence of cell selection by plant tissue culture media. In: White PR and Grove AR (eds) Proc Int Conf Plant Tissue Culture Penn State Univ. Berkely, CA: McCutchan, pp 473–484
Tsurumi S and Ohwaki Y (1978) Transport of 14C-labeled indoleacetic acid in Vicia root segment. Plant Cell Physiol 19: 1195–1206
Trewavas AJ (1976) Plant growth substances In: Bryant JA (ed) Molecular Aspects of Gene Expression in Plants. London: Academic Press, pp 249–299
Trewavas AJ (1979) The dynamics of meristem control by growth substances. In: George E (ed) Brit Plant Gr Reg Group Mono. 3. Differentiation and the Control of Development in Plants. Long Ashton, Bristol: British Plant Growth Regulator Group, pp 39–57
Trewavas AJ (1982) Growth substance sensitivity: The limiting factor in plant development. Physiol Plant 55: 60–72
Trewavas AJ (1985) Growth substances, calcium and the regulation of the cell cycle. In: Bryant JA and Francis D (eds) The Cell Division Cycle in Plants. Cambridge: Cambridge Univ Press, pp 133–156
Vantard M, Stoppin V and Lambert A-M (1998) Cell cycle dependent nucleation and assembly of plant microtubular proteins. In: Francis D, Dudits D and Inze D (eds) Plant Cell Division. Colchester UK: Portland Press, pp 301–318
Van't Hof J (1966) Experimental control of DNA synthesis and dividing cells in excised root tips of Pisum. Am J Bot 53: 970–976
Van't Hof J (1973) The regulation of cell division in higher plants. Brookhaven Symp 25: 152-165
Van't Hof J (1980) Pea (Pisum sativum) cells arrested in G2 have nascent DNA with breaks between replicons and replication clusters. Exp Cell Res 129: 231–237
Van't Hof J (1985) Control points within the cell cycle. In: Bryant JA and Francis D(eds) The Cell Division Cycle in Plants. Cambridge: Cambridge Univ Press, pp 1–13
Van't Hof J (1987) Functional chromosomal structure: the replicon. In: Bryant J and Dunham VL (eds) DNA replication in Plants. Boca Raton, Florida: CRC Press, pp 1–16
Van't Hof, J, Hoppin DP and Yagi S (1973) Cell arrest in G1 and G2 of the mitotic cell cycle of Vicia faba root meristems. Am J Bot 60: 889–895
Wang H, Fowke LC and Crosby WL (1997) A plant cyclindependent kinase inhibitor gene. Nature 386: 451
Webster PL and Van't Hof J (1969) Dependence on energy and aerobic metabolism of initiation of DNA synthesis and mitosis by G1 and G2 cells. Exp Cell Res 5: 88–94
Weinberg RA (1995) The retinoblastoma protein and cell cycle control. Cell 81: 323–330
Yeoman MM and Mitchell JP (1970) Changes accompanying the addition of 2,4-D to excised jerusalem artichoke tuber tissue. Ann Bot 34: 799–810
Zhang K, Letham DS and John PCL (1996) Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone0 kinase. Planta 200: 2–12
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Francis, D., Sorrell, D.A. The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regulation 33, 1–12 (2001). https://doi.org/10.1023/A:1010762111585
Issue Date:
DOI: https://doi.org/10.1023/A:1010762111585