Abstract
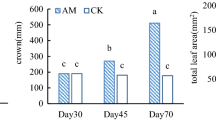
Lychee (Litchi chinensis Sonn.) is typically propagated by air-layering mature tree branches which are potted in fertilized, soil-free media after cutting. The size of these branches, low phosphorus retention by pot substrates, and fertilization all might combine to preclude benefits of arbuscular mycorrhizas to lychee. In order to examine the potential of lychee to benefit from arbuscular mycorrhizas in an agriculturally realistic context, lychee air-layers were grown for 469 days in ca. 95-l pots of soil-free substrate inoculated with field-collected arbuscular mycorrhizal roots or not at two different levels of phosphorus fertilization. High phosphorus fertilization (a one-time addition of ca. 1.32 g l−1 slow-release triple-superphosphate) had no detectable effects on mycorrhiza formation, lychee survival, net CO2 assimilation, or growth. Inoculation with indigenous South Florida arbuscular mycorrhizal fungi improved leaflet expansion as early as 120 days after inoculation, and subsequently enhanced height growth and leaf production but did not affect stem diameter growth, net CO2 assimilation, or survival. At harvest, although mycorrhizal colonization was low (average 7.4% colonized root length), mycorrhizal plants had 39% higher above-ground dry weight than control plants. Below-ground dry weights did not differ, but inoculated plants had lower fine root to leaf dry weight ratios than control plants. Leaflets of inoculated plants had higher concentrations of P, K, Cu, and Zn, and lower concentrations of Ca, Mg, and Mn than those of control plants, but total Kjeldahl nitrogen and iron concentrations did not differ significantly 10 months after inoculation. Mycorrhiza enhancement of lychee growth occurred even though phosphorus clearly was not limiting for growth. Our observations suggest that in this soil-free medium, arbuscular mycorrhizal fungus enhancement of copper and iron nutrition improved lychee growth.
Similar content being viewed by others
References
Bakarr M I 1997 Assessment of arbuscular mycorrhiza inoculum potential. PhD Dissertation. University of Miami, Coral Gables, FL, USA. 114 p.
Baylis G T S, McNabb R F R and Morrison T M 1963 The mycorrhizal nodules of podocarps. Trans. Br. Mycol. Soc. 46, 378–384.
Bethlenfalvay G J and Franson R L 1989 Manganese toxicity alleviated by mycorrhizae in soybean. J. Plant Nutr. 12, 953–970.
Biermann B and Linderman R G 1983 Effect of container plant growth medium and fertilizer phosphorus on establishment and host growth response to vesicular-arbuscular mycorrhizae.J. Am. Soc. Hort. Sci. 108, 962–971.
Buckman H O and Brady N C 1969 The Nature and Properties of Soils. Macmillan, New York, NY. 653 p.
Caris C, Hördt W, Hawkins H-J, Römheld V and George E 1998 Studies of iron transport by arbuscular mycorrhizal hyphae from soil to peanut and sorghum plants. Mycorrhiza8, 35–39.
Chang K and Chien K S 1990 VA mycorrhizae of citrus seedlings inoculated with Glomus epigaeum and its growth effects. Agric. Ecosyst. Environ. 29, 35–38.
Clark R B and Zeto S K 1996a Iron acquisition by mycorrhizal maize grown on alkaline soil. J. Plant Nutr.19, 247–264.
Clark R B and Zeto S K 1996b Mineral acquisition by mycorrhizal maize grown on acid and alkaline soil. Soil Biol. Biochem. 28, 1495–1503.
Clark R B and Zeto S K 2000 Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 23, 867–902.
Cooperband L R, Boerner R E J and Logan T J 1994 Humid tropical leguminous tree and pasture grass responsiveness to vesiculararbuscular mycorrhizal infection. Mycorrhiza4, 233–239.
Coville F V 1921 The lychee (Litchi chinensis) a mycorrhizal plant. Appendix VI. In The Lychee and Lungan. Ed. G W Groff. pp 151–152. Canton Christian College/Orange Judd Company, New York.
Cress WA, Johnson G V and Barton L L 1986 The role of endomycorrhizal fungi in iron uptake by Hilaria jamesii. J. Plant Nutr.9, 547–556.
Degner R L, Moss S D and Mulkey W D 1977 Economic Impact of Agriculture and Agribusiness in Dade County, Florida. FAMRC Industry Report 97–1. University of Florida, Gainesville, FL, USA. 74 p.
Estrada-Luna A A, Davies F T Jr. and Egilla J N 2000 Mycorrhizal fungi enhancement of growth and gas exchange of micropropagated guava plantlets (Psidium guajava L.) during ex vitro acclimatization and plant establishment. Mycorrhiza 10, 1–8.
Faber B A, Zasoski R J, Burau R G and Uriu K 1990 Zinc uptake by corn as affected by vesicular-arbuscular mycorrhizae. Plant Soil 129, 121–130.
Gerdemann J W 1968 Vesicular-arbuscular mycorrhiza and plant growth. Annu. Rev. Phytopathol.6, 397–418.
Gemma J N and Koske R E 1997 Arbuscular mycorrhizae in sand dune plants of the North Atlantic coast of the U.S., field and greenhouse inoculation and presence of mycorrhizae in planting stock. J. Environ. Manage.50,251–264.
Gemma J N, Koske R E, Roberts E M, Jackson N and DeAntonis K 1997 Mycorrhizal fungi improve drought resistance in creeping bentgrass. J. Turfgrass Sci.73, 15–29.
Getz R 1979 Florida daily temperature normal. Circular 464. University of Florida, Cooperative Extension Service, IFAS, Gainesville, FL, USA.41 p.
Giovannetti M and Mosse B 1980 An evaluation of techniques for measuring vesicular-arbuscular infection in roots. New Phytol. 84, 489–500.
Graham J H and Timmer L W 1984 Vesicular-arbuscular mycorrhizal development and growth response of rough lemon in soil and soilless media: effect of phosphorus source. J. Am. Soc. Hort. Sci. 109, 118–121.
Graham J H and Timmer L W 1985 Rock phosphate as a source of phosphorus for vesicular-arbuscular mycorrhizal development and growth of citrus in a soilless medium. J. Am. Soc. Hort. Sci. 110, 489–492.
Habte M and Byappanahalli M N 1994 Dependency of cassava (Manihot esculanta Crantz) on vesicular-arbuscular mycorrhizal fungi. Mycorrhiza4, 241–245.
Hanlon E A, Gonzales J S and Bartos J M 1994 IFAS Extension Soil Testing Laboratory Chemical Procedures and Training Manual.Circular812. University of Florida Cooperative Extension Service, IFAS, Gainesville, FL, USA. 76 p.
Janos D P 1980 Vesicular-arbuscular mycorrhizae affect lowland tropical rain forest plant growth. Ecology 61, 151–162.
Johnson C R, Joiner J N and Crews C E 1980 Effects of N, K and Mg on growth and leaf nutrient composition of three container grown woody ornamentals inoculated with mycorrhizae. J. Am. Soc. Hort. Sci. 105, 286–288.
Koske R E and Gemma J N 1989 A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 92, 486–488. 94
Koske R E, Gemma J N and Mueller W C 1989 Observations on 'sporocarps' of the VA mycorrhizal fungus Rhizophagus litchii. Mycol. Res.92, 488–490.
Larson K D, Schaffer B and Davies F S 1991 Flooding, leaf gas exchange, and growth of mango in containers. J. Am. Soc. Hort. Sci. 116, 156–160.
Liu A, Hamel C, Hamilton R I, Ma B L and Smith D L 2000 Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels.Mycorrhiza 9, 331–336.
Marschner H and Dell B 1994 Nutrient uptake in mycorrhizal symbiosis.Plant Soil159, 89–102.
Menzel C M and Simpson D R 1987 Lychee nutrition: a review.Sci. Hortic. 31, 195–224.
Menzel C M and Simpson D R 1994 Lychee. In Handbook of Environmental Physiology of Fruit Crops, Vol. 2, Sub-tropical and Tropical Crops. Eds. B Schaffer and P C Andersen. pp 123–145. CRC Press, Boca Raton, FL, USA.
Menzel C M, Carseldine M L, Haydon G F and Simpson D R 1992 A review of existing and proposed new leaf nutrient standards for lychee.Sci. Hortic.49,33–53.
Menzel C M, Rasmussen T S and Simpson D R 1995 Carbohydrate reserves in lychee trees (Litchi chinensis Sonn.).J. Hort. Sci. 70, 245–255.
NOAA 1999 Web site. http://www.ncdc.noaa.gov/ol/climate/cli matedata.html
Pandey S P and Misra A P 1971 Rhizophagus in mycorrhizal association with Litchi chinensis Sonn.Mycopathol. Mycol. Appl.45, 337–354.
Pandey S P and Misra A P 1975 Mycorrhiza in relation to growth and fruiting of Litchi chinensis Sonn.J. Indian Bot. Soc.54, 280–293.
Powell C Ll, Bagyaraj D J, Clark G E and Caldwell K I 1985 Inoculation with vesicular-arbuscular mycorrhizal fungi in the greenhouse production of asparagus seedlings.N.Z. J. Agric. Res. 28, 293–297.
Read D 1993 Mycorrhizas. Appendix C. In Tropical Soil Biology and Fertility. Eds. J M Anderson and J S I Ingram. pp 121–131. CAB International, Wallingford, Oxon, UK.
Same B I, Robson A D and Abbott L K 1983 Phosphorus, soluble carbohydrates and endomycorrhizal infection [Trifolium subterraneum]. Soil Biol. Biochem. 15,593–597.
Scheiner S M 1993 MANOVA: Multiple response variables and multispecies interactions. Chapter 5. In Design and Analysis of Ecological Experiments. Eds. S M Scheiner and J Gurevitch. pp 94–112. Chapman and Hall, New York, USA.
Sylvia DM and Jarstfer A G 1992 Sheared-root inocula of vesiculararbuscular mycorrhizal fungi.Appl. Environ. Microbiol.58, 229–232.
Treeby M T 1992 The role of mycorrhizal fungi and nonmycorrhizal micro-organisms in iron nutrition of citrus. Soil Biol. Biochem. 24, 857–864.
Wilkinson L 1990 SYSTAT: The system for statistics.SYSTAT, Inc., Evanston, USA. 677 p.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Janos, D.P., Schroeder, M.S., Schaffer, B. et al. Inoculation with arbuscular mycorrhizal fungi enhances growth of Litchi chinensis Sonn. trees after propagation by air-layering. Plant and Soil 233, 85–94 (2001). https://doi.org/10.1023/A:1010329618152
Issue Date:
DOI: https://doi.org/10.1023/A:1010329618152