Abstract
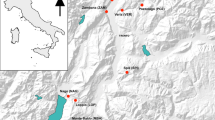
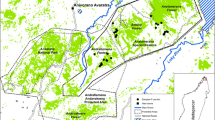
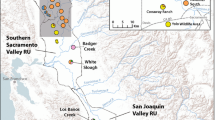
Analysis of 155 individuals with seven polymorphicmicrosatellite DNA markers showed significant genetic differentiationbetween the only three remaining subpopulations of the globally,critically-endangered Taita thrush. Small, recently-disturbedsubpopulations such as studied here may violate the assumptions ofmutation-drift and gene flow-drift equilibrium inherent to mostpopulation genetic tools that estimate gene flow. We thereforeidentified putative dispersers using two recently-developed assignmenttests based on individual genotypes. Previous-generation and currentmigration rates between any two subpopulations were estimated at one andzero individuals per generation, respectively. Strong congruence withnon-genetic estimates of between-fragment dispersal provided indirectevidence for the accuracy of the assignment test. From a conservationperspective, the available demographic and genetic data suggest asubstantial threat to the long-term survival of at least the smallestsubpopulation.
Similar content being viewed by others
References
Beentje HJ (1987) An ecological and floristic study of the forests of the Taita Hill, Kenya. Utafiti, 1, 23–66.
Beerli P, Felsenstein J (1999) Maximum-likelihood estimation of migration rates and population numbers of two populations using a coalescent approach. Genetics, 152, 763–773.
Brooks T, Lens L, Barnes J, Barnes R, Kihuria JK, Wilder C (1998) The conservation status of the forest birds of the Taita Hills, Kenya. Bird Conserv. Intern., 8, 119–139.
Caro TM, Laurenson MK (1994) Ecological and genetic factors in conservation: a cautionary tale. Science, 263, 485–486.
Collar NJ, Stattersfield AJ, Crosby MJ (1994) Birds to Watch 2. The World List of Threatened Birds. BirdLife Conserv. Ser. No. 4. Cambridge, U.K., BirdLife International.
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics, 144, 2001–2014.
Dallimer M (1999) Cross-species amplification success of avian microsatellites in the redbilled quelea Quelea quelea. Mol. Ecol., 8, 685–702.
Davies N, Villablanca FX, Roderick GK (1999) Determining the source of individuals: multilocus genotyping in nonequilibrium population genetics. TREE, 14, 17–21.
Degnan SM, Robertson BC, Clegg SM, Moritz CC (1999) Microsatellite primers for studies of gene flow and mating systems in white-eyes (Zosterops). Mol Ecol., 8, 159–160.
Ellegren H, Lifjeld JT, Slagsvold T, Primmer CR (1995) Handicapped males and extrapair paternity in pied flycatchers: a study using microsatellite markers. Mol. Ecol., 4, 739–744.
Frankham R (1995a) Conservation genetics. Annu. Rev. Genetics, 29, 305–327.
Frankham R (1995b) Effective population size/adult population size ratios in wildlife: a review. Genet. Res. Camb., 66, 95–107.
Frankham R (1998) Inbreeding and extinction: island populations. Conservation Biology, 12, 665–675.
Frankel OH, Soulé ME (1981) Conservation and Evolution. Cambridge University Press, New York, NY, USA.
Franklin IR (1980) Evolutionary change in small populations. In: Conservation Biology: An Evolutionary Approach (eds. Soulé ME, Wilcox, BA), pp. 135–149. Sinauer, Sunderland, MA.
Franklin IR, Frankham R (1998) How large must populations be to retain evolutionary potential? Animal Conservation, 1, 69–73.
Gaggiotti OE, Lange O, Rassmann K, Gliddon CA (1999) Comparison of two indirect methods for estimating average levels of gene flow using microsatellite data. Mol. Ecol., 8, 1513–1520.
Goodman SJ (1997) RstCalc: a collection of computer programs for calculating estimates of genetic differentiation from microsatellite data and determining their significance. Mol. Ecol., 6, 881–885.
Griffiths R, Double MCY, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol. Ecol., 7, 1071–1075.
Guo SW, Thompson EA (1992) Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics, 48, 361–372.
Haig SM, Grattto-Trevor CL, Mullins TD, Colwell MA (1997) Population identification of western hemisphere shorebirds throughout the annual cycle. Mol. Ecol., 6, 412–427.
Hartl DL, Clarl AG (1989) Principles of Population Genetics. Sinauer Associates Inc, Sunderland, MA.
Hedgecock D, Sly F (1990) Genetic drift and effective population sizes of hatchery-propagated stocks of the Pacific oyster, Crassostrea gigas. Aquaculture, 88, 21–38.
Houlden BA, England PR, Taylor AC, Greville WD (1996) Low genetic variability of the koala Phascolarctos cinereus in southeastern Australia following a severe population bottleneck. Mol. Ecol., 5, 269–281.
Lande R, Barrowclough GF (1987) Effective population size, genetic variation, and their use in population management. In: Viable Populations for Conservation. (ed. Soulé ME), pp. 87–123. Cambridge University Press, New York.
Leberg PL (1992) Effects of population bottlenecks on genetic diversity as measured by allozyme electrophoresis. Evolution, 46, 477–494.
Lens L, Adriaensen F, Matthysen E (1999a) Dispersal studies in recently and historically fragmented forests-a comparison between Kenya and Belgium. In: Proc. 22nd Int. Ornithol. Congr., Durban (eds. Adams N, Slotow R) pp. 2480–2491. University of Natal, Natal.
Lens L, Galbusera P, Brooks T, Waiyaki E, Schenck T (1998) Highly skewed sex ratios in the critically endangered Taita Thrush as revealed by CHD genes. Biodiversity and Conservation, 7, 869–873.
Lens L, Van Dongen S (1999) Evidence for organism-wide asymmetry in five bird species of a fragmented afrotropical forest. Proc. R. Soc. Lond. B., 266, 1055–1060.
Lens L, Van Dongen S, Wilder CM, Brooks TM, Matthysen E (1999b) Fluctuating asymmetry increases with habitat disturbance in seven bird species of a fragmented afrotropical forest. Proc. R. Soc. Lond. B., 266, 1241–1246.
Lewis PO, Zaykin D (1999) Genetic Data Analysis: Computer Program for the Analysis of Allelic Data. Version 1.0 (d12). Free program distributed by the authors over the internet from the GDA Home Page at <http://chee.unm.edu/gda/>
Li Shou-Hsien, Yi-Jiun Huang, Brown JL (1997) Isolation of tetranuleotide microsatellites from the Mexican jay Aphelocoma ultramarina. Mol. Ecol., 6, 499–501.
Lovett J (1985) Moist forests of Eastern Tanzania. Swara, 8, 8–9.
Luikart G, England PR (1999) Statistical analysis of microsatellite DNA data. TREE, 14, 253–256.
McCommas SA, Bryant EH (1990) Loss of electrophoretic variation in serially bottlenecked populations. Heredity, 64, 315–321.
McDonald DB, Potts W(1994) Cooperative display and relatedness among males in a lek-mating bird. Science, 266, 1030–1032.
Mills LS, Allendorf FW (1996) The one-migrant-per-generation rule in conservation and management. Conservation Biology, 10, 1509–1518.
Nei M, Graur D (1984) Extent of protein polymorphism and the neutral mutation theory. Evolutionary Biology, 17, 73–118.
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution, 29, 1–10.
Michalakis Y, Excoffier L (1996) A generic estimation of population subdivision using distances between alleles with special reference for microsatellite loci. Genetics, 142, 1061–1064.
Petren K (1998) Microsatellite primers from Geospiza fortis and cross-species amplification in Darwin's finches. Mol. Ecol., 7, 1771–1788.
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics (in press).
Rannala B, Mountain J (1997) Detecting immigration by using multilocus genotypes. Proc. Natl. Acad. Sci. USA, 94, 9197–9201.
Raymond M, Rousset (1995a) Genepop (Version1.2): population genetics software for exact tests and ecumenicism. The Journal of Heredity, 86, 248–249.
Raymond M, Rousset F (1995b) An exact test for population differentiation. Evolution, 49, 1280–1283.
Rice WR (1989) Analyzing tables of statistical tests. Evolution, 43, 223–225.
Saino N, Primmer CR, Ellegren H, Pape Moller A (1997) An experimental study of paternity and tail ornamentation in the barn swallow (Hirundo rustica). Evolution, 51, 562–570.
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. pp. 9.14–9.23. Cold Spring Harbour Press, New York
Schonewald-Cox CM, Chambers SM, Mac-Bryde B, Thomas, WL (1983) Genetics and Conservation: A Reference for Managing Wild Animal and Plant Populations. Benjamin Cummings, Menlo Park, CA.
Schneider S, Roessli D, Excoffier L (1999) Arlequin ver. 2.0: A Software for Population Genetic Data Analysis. Genetics and Biometry Laboratory, University of Geneva, Switzerland.
Simberloff, DS (1988) The contribution of population and community biology to conservation science. Annu. Rev. Syst. Ecol., 19, 473–511.
Slatkin M (1995) A measure of population subdivision based on microsatellite allele frequencies. Genetics, 139, 457–462.
Tarr CL, Fleischer RC (1999) Population boundaries and genetic diversity in the endangered Mariana crow (Corvus kubaryi). Mol. Ecol., 8, 941–949.
Walsh PS, Metzger DA, Higuchi R (1991) Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques, 10, 506–513.
Waser PM, Strobeck C (1998) Genetic signatures of interpopulation dispersal. TREE, 13, 43–44.
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution, 38, 1358–1370.
Wilder CM, Brooks TM, Lens L (1999) Vegetation structure and composition of the Taita Hills forests. J. E. Afr. Nat. Hist. Soc. (in press).
Wright S (1931) Evolution in Mendelian populations. Genetics, 16, 97–259.
Wright S (1951) The genetical structure of populations. Annals of Eugenics, 15, 323–354.
Wright S (1977) Evolution and the Genetics of Populations. Univ. of Chicago Press, Chicago.
Zimmerman DA, Turner DA, Pearson DJ (1996) Birds of Kenya and northern Tanzania. Christopher Helm, London.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galbusera, P., Lens, L., Schenck, T. et al. Genetic variability and gene flow in the globally, critically-endangered Taita thrush. Conservation Genetics 1, 45–55 (2000). https://doi.org/10.1023/A:1010184200648
Issue Date:
DOI: https://doi.org/10.1023/A:1010184200648