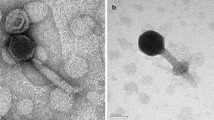
Abstract
The cyanophage AN-15 was found to have a requirement for either 1 mM calcium or 1 mM magnesium ions to maintain viral stability, whereas 1 mM calcium ions alone were essential for the infection process to proceed in Anabaena sp. strain PCC 7120. Following prolonged incubation, phage-resistant cells were detected at a high frequency (approximately 10-5) in lysates, as either renewed growth in liquid cultures, or as colonies in confluently lysed lawns. Southern hybridisation failed to detect AN-15 DNA in any of the resistant strains, implying that resistance is unlikely to be due to the presence of temperate phages. A high rate of spontaneous mutation is therefore likely to be the cause of resistance. Two classes of resistant cells were identified; those in which AN-15 failed to attach to host cells, and those in which attachment occurred, but subsequent replication was defective. However, it was possible to overcome phage resistance by the isolation of spontaneous mutants of AN-15, capable of infecting phage-resistant cells. These observations imply that if cyanophages are to be assessed as a means of controlling cyanobacterial blooms in freshwater bodies, the ionic (notably calcium) concentration of the water must be considered, together with the possible need to employ alternative cyanophage strains if resistance to the original one arises.
Similar content being viewed by others
References
Adams DG (1988) Isolation and restriction analysis of DNA from heterocysts and vegetative cells of cyanobacteria. J. gen. Microbiol. 134: 2943–2949.
Bancroft I (1986) PhD Thesis. Lancaster University, UK
Bancroft I, Smith RJ (1988) The isolation of genomic DNA from cyanophage infecting Nostoc and Anabaena species of cyanobacteria. New Phytol. 110: 233–239.
Barnet Y, Daft MJ, Stewart WDP (1981) Cyanobacteria-cyanophage interactions in continuous culture. J. appl. Bact. 51: 541–552.
Brabrand A, Faafeng BA, Källqvist T, Nilssen JP (1983) Biological control of undesirable cyanobacteria in culturally eutrophic lakes. Oecologia 60: 1–5.
Carmichael WW (1992) Cyanobacteria secondary metabolites - the cyanotoxins. J. appl. Bact. 72: 445–459.
Desjardins PR, Olson GB (1983) Viral control of nuisance cyanobacteria (blue-green algae). California Water Resources Center, Contribution 185: 1–35.
Hayes W (1964) The Genetics of Bacteria and their Viruses. Black Sci. Publ., Oxford.
Hennes KP, Suttle CA, Chan AM (1995) Fluorescently labeled virus probes show that natural virus populations can control the struc-ture of marine microbial communities. Appl. envir. Microbiol. 61: 3623–3627.
Hu N-T, Theil T, Giddings TH Jr., Wolk CP (1981) New Anabaena and Nostoc cyanophages from sewage settling ponds. Virology 114: 236–246.
Martin E (1982) Biological regulation of bloom-causing blue-green algae: A feasible alternative. Nebraska Water Resources Completion Report A-056: 1–36.
Mole RJ (1994) PhD. thesis. University of Leeds, UK.
National Rivers Authority (1990) Toxic blue-green algae. National Rivers Authority, Peterborough, UK.
Ohki K, Fujita Y (1996) Occurrence of a temperate cyanophage lysogenizing the marine cyanophyte Phormidium persicinum. J. Phycol. 32: 365–370.
Oliveira AR, Mudd JB, Desjardins PR (1982). AS-1 cyanophage adsorption to liposomes. J. gen. Virol. 61: 153–156.
Phlips EJ, Monegue RL, Aldridge FJ (1990) Cyanophages which impact bloom-forming cyanobacteria. J. aquat. Plant Mgmt 28: 92–97.
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343: 60–62.
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. gen. Microbiol. 111: 1–61.
Safferman RS, Morris ME (1964) Control of algae with viruses. J. am. Wat. Works Ass. 56: 1217–1224.
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Samimi B, Drews G (1978) Adsorption of cyanophage AS-1 to unicellular cyanobacteria and isolation of receptor material from Anacystis nidulans.J. Virol. 25: 164–174.
Sarma TA, Kaur B (1993) Spontaneous and induced host-range mutants of cyanophage N-1. Arch. Virol. 130: 195–200.
Sinha NK, Goodman MF (1983) Fidelity of DNA Replication. In Mathews CK, Kutter EM, Mosig G, Berget PB (eds), Bacteriophage T4. Am. Soc. Microbiol., Washington D.C.: 131–137.
Suttle CA (in press) Cyanophages and their role in the ecology of cyanobacteria. In Whitton BA, Potts M (eds), Ecology of Cyanobacteria: their Diversity in Time and Space. Kluwer Academic Publishers, Dordrecht.
Suttle CA, Chan AM (1994) Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl. envir. Microbiol. 60: 3167–3174.
Suttle CA, Chan AM, Cottrell MT (1990) Infection of phytoplankton by viruses and reduction of the primary productivity. Nature 347: 467–469.
Waterbury JB, Valois FW (1993) Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl. envir. Microbiol. 59: 3393–3399.
Weinbauer Mg, Suttle CA (1996) Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico. Appl. envir. Microbiol. 62: 4374–4380.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mole, R., Meredith, D. & Adams, D.G. Growth and phage resistance of Anabaena sp. strain PCC 7120 in the presence of cyanophage AN-15. Journal of Applied Phycology 9, 339–345 (1997). https://doi.org/10.1023/A:1007938624025
Issue Date:
DOI: https://doi.org/10.1023/A:1007938624025