Abstract
Purpose. To use artificial neural networks for predicting dissolution profiles of matrix-controlled release theophylline pellet preparation, and to evaluate the network performance by comparing the predicted dissolution profiles with those obtained from physical experiments using similarity factor.
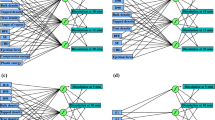
Methods. The Multi-Layered Perceptron (MLP) neural network was used to predict the dissolution profiles of theophylline pellets containing different ratios of microcrystalline cellulose (MCC) and glyceryl monostearate (GMS). The concepts of leave-one-out as well as a time-point by time-point estimation basis were used to predict the rate of drug release for each matrix ratio. All the data were used for training, except for one set which was selected to compare with the predicted output. The closeness between the predicted and the reference dissolution profiles was investigated using similarity factor (f 2).
Results. The f 2 values were all above 60, indicating that the predicted dissolution profiles were closely similar to the dissolution profiles obtained from physical experiments.
Conclusion. The MLP network could be used as a model for predicting the dissolution profiles of matrix-controlled release theophylline pellet preparation in product development.
Similar content being viewed by others
REFERENCES
J. G. Taylor. The Promise of Neural Networks, Springer-Verlag, London (1993).
J. Takahara, K. Takayama, and T. Nagai. Multi-objective simultaneous optimization based on artificial neural network in sustained released formulations. J. Contr. Rel. 49:11-20 (1997).
J. Takahara, K. Takayama, K. Isowa, and T. Nagai. Multi-objective simultaneous optimization based on artificial neural network in a ketoprofen hydrogel formula containing O-ethyl-menthol as a percutaneous absorption enhancer. Int. J. Pharm. 15:203-210 (1997).
A. S. Hussian, X. Yu, and R. D. Johnson. Application of neural computing in pharmaceutical product formulation. Pharm. Res. 8:1248-1252 (1991).
A. S. Hussian, R. D. Johnson, N. Vachhrajani, and W. A. Ritshel. Feasibility of developing a neural network for prediction of human pharmacokinetic parameters from animal data. Pharm. Res. 10:466-469 (1993).
E. Brier, J. M. Zurada, and G. R. Aronoff. Neural network predicted peak and trough of gentamicin concentrations. Pharm. Res. 12:406-412 (1995).
D. E. Rumelhart, G. E. Hinton, and R. J. Williams. Learning internal representation by error propagation. In D. E. Rumelhart and J. L. McLelland (eds.), Parallel Distributed Processing, I. MIT Press, Cambridge, MA, 1986. pp. 318-362.
G. Cybenko. Approximation by superposition of a sigmoidal function. Mathematics of Control, Signals and Systems. 2:303-314 (1989).
FDA Guidance for Industry: Immediate release solid dosage forms: Scale-up and Post Approval Changes (SUPAC-IR): Chemistry, Manufacturing and Controls. In vitro dissolution testing and in vivo bioequivalence documentation, November, 1995.
FDA Guidance for Industry: Dissolution testing of immediate release solid oral dosage forms, August, 1997.
FDA Guidance for Industry: Extended release solid oral dosage forms: Development, evaluation and application of in vitro/in vitro correlations, September, 1997.
FDA Guidance for Industry: Modified release solid dosage forms: Scale-up and Post Approval Changes (SUPAC-MR): Chemistry, Manufacturing and Controls. In vitro dissolution testing and in vivo bioequivalence documentation, September, 1997.
K. K. Peh and K. H. Yuen. Development and in vitro evaluation of a novel multi-particulate matrix controlled release formulation of theophylline. Drug Dev. Ind. Pharmacy 21:1545-1555 (1995).
T. Masters. Practical Neural Network Recipes in C++: Academic Press, San Diego, CA, 1993.
B. F. J. Manly. Multivariate statistical methods: A primer. Chapman & Hall, New York, 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Peh, K.K., Lim, C.P., Quek, S.S. et al. Use of Artificial Neural Networks to Predict Drug Dissolution Profiles and Evaluation of Network Performance Using Similarity Factor. Pharm Res 17, 1384–1389 (2000). https://doi.org/10.1023/A:1007578321803
Issue Date:
DOI: https://doi.org/10.1023/A:1007578321803