Abstract
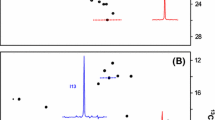
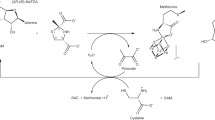
Methionyl-tRNA synthetase (MetRS) from Bacillus stearothermophilus was shown to undergo covalent methionylation by a donor methionyl-adenylate, the mixed carboxylic-phosphoric acid anhydride synthesized by the enzyme itself. Covalent reaction of methionyl-adenylate with the synthetase or other proteins proceeds through the formation of an isopeptide bond between the carboxylate of the amino acid and the ∈-NH2 group of lysyl residues. The stoichiometries of labeling, as followed by TCA precipitation, were 2.2 ± 0.1 and 4.3 ± 0.1 mol of [14C]Met incorporated by 1 mol of the monomeric MS534 and the native dimeric species of B. stearo methionyl-tRNA synthetase, respectively. Matrix-assisted laser desorption-ionization mass spectrometry designated lysines-261, -295, -301 and -528 (or -534) of truncated methionyl-tRNA synthetase as the target residues for covalent binding of methionine. By analogy with the 3D structure of the monomeric M547 species of E. coli methionyl-tRNA synthetase, lysines-261, -295, and -301 would be located in the catalytic crevice of the thermostable enzyme where methionine activation and transfer take place. It is proposed that, once activated by ATP, most of the methionine molecules react with the closest reactive lysyl residues.
Similar content being viewed by others
REFERENCES
Blanquet, S., Fayat, G., and Waller, J. P. (1974). Eur. J. Biochem. 44, 343–351.
Cusack, S., Berthet-Colominas, C., Härtlein, M., Nassar, N., and Leberman, R. (1990). Nature 347, 249–255.
Eriani, G., Delarue, M., Poch, O., Gangloff, J., and Moras, D. (1990). Nature 347, 203–206.
Fourmy, D., Mechulam, Y., Brunie, S., Blanquet, S., and Fayat, G. (1991). FEBS Lett. 292, 259–263.
Ghosh, G., Pelka, H., Schulman, L. D., and Brunie, S. (1991). Biochemistry 30, 9569–9575.
Gillet, S., Hountondji, C., Schmitter, J. M., and Blanquet, S. (1997). Prot. Sci. 6, 2426–2435.
Guillon, J. M., Meinnel, T., Mechulam, Y., Lazennec, C., Blanquet, S., and Fayat, G. (1992). J. Mol. Biol. 224, 359–367.
Hountondji, C., Blanquet, S., and Lederer, F. (1985). Biochemistry 24, 1175–1180.
Hountondji, C., Lederer, F., Dessen, P., and Blanquet, S. (1986a). Biochemistry 25, 16–21.
Hountondji, C., Dessen, P., and Blanquet, S. (1986b). Biochimie 68, 1071–1078.
Hountondji, C., Schmitter, J. M., Fukui, T., Tagaya, M., and Blanquet, S. (1990). Biochemistry 29, 11266–11273.
Kalogerakos, T., Hountondji, C., Berne, P. F., Dutka, S., and Blanquet, S. (1994). Biochimie 76, 33–44.
Kalogerakos, T., Dessen, P., Fayat, G., and Blanquet, S. (1980). Biochemistry 19, 3712–3723.
Kern, D., Lorber, B., Boulanger, Y., and Giegé, R. (1985). Biochemistry 24, 1321–1332.
Lawrence, F., Blanquet, S., Poiret, S., Robert-Gero, M., and Waller, J. P. (1973). Eur. J. Biochem. 36, 234–243.
Mechulam, Y., Dardel, F., Le Corre, D., Blanquet, S., and Fayat, G. (1991). J. Mol. Biol. 217, 465–475.
Mechulam, Y., Schmitt, E., Maveyraud, L., Zelwer, C., Nureki, O., Yokoyama, S., Konno, M., and Blanquet, S. (1999). J. Mol. Biol. 294, 1287–1297.
Meinnel, T., Mechulam, Y., and Fayat, G. (1988). Nucleic Acid Res. 16, 8095–8096.
Mejdoub, H., Kern, D., Giegé, R., Ebel, J. P., Boulanger, Y., and Reinbolt, J. (1987). Biochemistry 26, 2054–2059.
Nakajima, H., Kitabatake, S., Tsurutani, R., Yamamoto, K., Tomioka, I., and Imahori, K. (1986). Int. J. Peptide Protein Res. 28, 179–185.
Rapaport, E., Yogeeswaran, G., Zamecnik, P. C., and Rémy, P. (1985). J. Biol. Chem. 260, 9509–9512.
Schmitt, E., Panvert, M., Mechulam, Y., and Blanquet, S. (1997). Eur. J. Biochem. 246, 539–547
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hountondji, C., Beauvallet, C., Pernollet, JC. et al. Enzyme-Induced Covalent Modification of Methionyl-tRNA Synthetase from Bacillus stearothermophilus by Methionyl-Adenylate: Identification of the Labeled Amino Acid Residues by Matrix-Assisted Laser Desorption-Ionization Mass Spectrometry. J Protein Chem 19, 563–568 (2000). https://doi.org/10.1023/A:1007194101107
Published:
Issue Date:
DOI: https://doi.org/10.1023/A:1007194101107