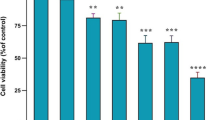
Abstract
Molecular mechanisms of lipid synthesis and their controls in hepatic stellate cells are not known. We have previously proposed that, in contrast to other fat storing cells, hepatic stellate cells are not involved in energy storage, but they represent a particular cell population specialized in storage of lipid-soluble substances, the major one being probably retinol. In agreement with this hypothesis, induction of the lipocyte phenotype in stellate cells is not under the control of insulin, but responds to retinoids and other molecules that modify the gene expression program in these cells. In the present study we have monitored the activity of the two major enzymes involved in lipid synthesis during the induction of the lipocyte phenotype in hepatic stellate cells: glycerol-3-phosphate dehydrogenase (GPDH) that mediates the de novo lipid synthesis, and lipoprotein lipase that mediates incorporation of plasma lipids. In early stages of lipocyte induction, both pathways of lipid synthesis are activated. When lipocytes have already constituted the lipid droplets, lipoprotein lipase pathway is downregulated, while GPDH activity remains high. Adult liver has been reported to lack lipoprotein lipase, but under stress, lipase activity was detected around and at the surface of the intrahepatic vasculature. We have now shown that the lipase activity can be induced in the hepatic stellate cells, located in the Disse's space. The high lipoprotein lipase activity under acute induction of lipocyte phenotype, followed by the low activity under conditions of metabolic equilibrium, are in compass with the increased activity of this enzyme under stress, and its low activity in adult liver parenchyma under normal conditions.
Similar content being viewed by others
References
Ailhaud G, Grimaldi P, Negrel R: Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr 12: 207–233,1992
Blomhoff R, Rasmussen M, Nilsson A, Norum KR, Berg T, Blaner WS, Kato M, Mertz, JR, Goodman, DSW, Eriksson, U, Peterson, PA: Hepatic retinol metabolism: distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J Biol Chem 260: 13560–13565, 1985
Mak KM, Leo MA, Lieber CS: Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology 87: 188–200, 1984
Voss B, Rauterberg J, Pott G, Brehmer U, Allan S, Lehmann R, Bassewitz DB von: Non-parenchymal cells cultivated from explants of fibrotic liver resemble endothelial and smooth muscle cells from vessel walls. Hepatology 2: 19–28, 1982
Borojevic R, Vinhas SA, Monteiro ANA, Domont GB, Zyngier FR, Grimaud JA: Liver connective tissue cells isolated from human schistosomal fibrosis or alcoholic cirrhosis represent a modified phenotype of smooth muscle cells. Biol Cell 53: 231–238, 1985
Rungger-Brandle E, Gabbiani G: The role of cytoskeletal and contractile elements in pathologic processes. J Pathol 110: 261–392, 1983
Gressner AM: Liver fibrosis: perspectives in pathobiochemical research and clinical outlook. Eur J Clin Chem Clin Biochem 29: 293–311,1991
Borojevic R, Monteiro ANA, Vinhas SA, Domont GB, Mourão PAS, Emonard H, G rimaldi GJ, Grimaud, JA: Establishment of a continuous cell line from fibrotic schistosomal granulomas in mice livers. In Vitro Cell Dev Biol 21: 382–390, 1985
Lazou JM, Zampetti-Bosseler F, Geerts A, Borojevic R, Wisse E: In: D.L. Knook, E. Wisse (eds). Tissue distribution of fat-storing cells in normal and Schistosoma mansoni infected mouse liver. Cells of the Hepatic Sinusoid. Leiden: The Kupffer Cell Foundation, 1993, pp 304–307
Boloukhère M, Baldo-Correa E, Borojevic R: Experimental murine schistosomiasis mansoni: characterization of connective tissue cells in hepatic periovular ganulomas. J Submicroscop Cytol Pathol 25: 505–517, 1993
Greenwel P, Rubin J, Schwartz M, Hertzberg EL, Rojkind M: Liver fat storing cell clones obtained from a ClC4-cirrhotic rat are heterogeneous with regard to proliferation, expression of extracellular matrix components, interleukin-6, and connexin 43. Lab Invest 69: 210–216, 1993
Inagaki Y, Truter S, Greenwel P, Rojkind M, Unoura M, Kobayashi K, Ramirez F: Regulation of the alpha 2(1) collagen gene transcription in fat-storing cells derived from a cirrhotic liver. Hepatology 22: 573–579, 1995
Margis R, Borojevic R: Retinoid-mediated induction of the fat-storing phenotype in a liver connective tissue cell line (GRX). Biochim Biophys Acta 1011: 1–5, 1989
Borojevic R, Guaragna RM, Margis R, Dutra HS: In vitro induction of the fat-storing phenotype in a liver connective tissue cell line – GRX. In Vitro Cell Dev Biol 25: 361–368, 1990
Williams IH, Polakis SE: Differentiation of 3T3-L1 fibroblasts to adipocytes: the effect of indomethacin, prostaglandin E1 and cyclic AMP on the process of differentiation. Biochem Biophys Res Commun 77: 175–186, 1977
Guaragna RM, Trugo L, Borojevic R: Neutral lipid synthesis and accumulation during in vitro induction of the lipocyte phenotype in hepatic connective tissue cells. Biochim Biophys Acta 1085: 29–34, 1991
Guaragna RM, Trugo L, Borojevic R: Phospholipid synthesis and phosphorylation during conversion of hepatic myofibroblasts into lipocytes (Ito cells). Biochim Biophys Acta 1128: 237–243, 1992
Yumoto S, Yumoto K, Yamamoto M: In: E. Wisse, D.L. Knook, K. Decker (eds). Effects of retinoic acid on Ito cells (fat storing cells) in vitamin A-deficient rats. Cells of the Hepatic Sinusoid. Rijswijk, Holland: The Kupffer Cell Foundation, 1988, pp 33–38
Ailhaud G: Adipose cell differentiation in culture. Mol Cell Biochem 49: 17–31, 1982
Bensadoun A: Lipoprotein lipase. Annu Rev Nutr 11: 217–237, 1991
Rea TJ, De Mattos RB, Pape ME: Hepatic expression of genes regulating lipid metabolism in rabbits. J Lipid Res 34: 1901–1910, 1993
Kirschgessner TG, Svenson KL, Lusis AJ, Schotz MC: The sequence of cDNA encoding lipoprotein lipase. A member of a lipase gene family. J Biol Chem 262: 8463–8466, 1987
Kozak LP, Jensen JT: Genetic and developmental control of multiple forms of l-glycerol 3-phosphate dehydrogenase. J Biol Chem 249: 7775–7781, 1974
Wise LS, Green H: Participation of one isoenzyme of cytosolic glycerolphosphate dehydrogenase in the adipose conversion of 3T3 cells. J Biol Chem 254: 273–275, 1979
Bradford MM: A rapid sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein binding dye. Anal Biochem 72: 248–250,1976
Spooner PM, Chernick SS, Garrison MM, Scow RO: Insulin regulation of lipoprotein lipase activity and release in 3T3-L1 adipocytes. J Biol Chem 254: 10021–10029, 1979
Nilsson-Ehle P, Shotz MC: A stable radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res 17: 536–545, 1976
Burton K: Determination of DNA concentration with diphenylamine. In: L. Grossman, K. Moldave (eds). Methods in Enzymology. New York: Academic Press, 1968, pp 163–166
Robinson DS, Speake BK: Role of insulin and other hormones in the control of lipoprotein lipase activity. Biochem Soc Trans 17: 40–42, 1989
Troen G, Nilsson A, Norum KR, Blomhoff R: Characterization of liver stellate cell retinyl ester storage. Biochem J 300: 793–798, 1994
Teboul M, Bismuth J, Gharbi-Chiri J, Valette A, Bonne J, Ghiringhelli O, Torresani J: Retinoic acid decreases nuclear triodothyronine receptor expression and impairs an early step of adipose differentiation in the thyroid hormone-sensitive mouse Ob 17 preadipocyte cell line. Endocrinology 130: 1475–1482, 1992
Kamea Y, Kawada T, Fujita A, Etinne J, Noe L, Sugimoto C: Lipoprotein lipase enzyme expression in 3T3-L1 adipocytes is posttranscriptionally down regulated by retinoic acid. Biochem Internat 26: 923–934, 1992
Oliver JD, Rogers MP: Effect of retinoic acid on lipoprotein lipase activity and mRNA level in vitro and in vivo. Biochem Pharmacol 45: 579–583, 1993
Wake K: Perisinusoidal stellate cells (fat-storing cells, interstitial cells, lipocytes), their related structures in and around the liver sinusoids, and vitamin A-storing cells in extrahepartic organs. Int Rev Cytol 66: 303–353, 1980
Bronfenmajer S, Schaffner F, Popper H: Fat-storing cells (lipocytes) in human liver. Arch Pathol 82: 447–453, 1966
Ballardini G, Groff P, De Giorgi LB, Schuppan D, Bianchi FB: Ito cell heterogeneity: desmin-negative Ito cells in normal rat liver. Hepatology 19: 440–446,1994
Ramm GA, Britton RS, O'Neill R, Blaner WS, Bacon BR: Vitamin A-poor lipocytes: a novel desmin-negative lipocyte cell population, which can be activated to myofibroblasts. Am J Physiol 269G: 532–541, 1995
Ramadori G: The stellate cell (Ito-cell, fat-storing cell, lipocyte, perisinusoidal cell) of the liver. New insights into pathophysiology of an intriguing cell. Virchows Arch B Cell Pathol 61: 147–158, 1991
Friedman SL: The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med 328: 1828–1835, 1993
Dani C, Doglio A, Amri E, Bardon S, Fort P, Bertrand B, Grimaldi P, Ailhaud G: Cloning and regulation of a mRNA specifically expressed in the preadipose state. J Biol Chem 264: 10119–10125, 1989
Zehner R, Moser R, Newman TC, Fried SK, Breslow JL: Apolipoprotein E gene expression in mouse 3T3-L1 adipocytes and human adipose tissue and its regulation by differentiation and lipid content. J Biol Chem 266: 10583–10588, 1991
Cisar LA, Hoogewerf AJ, Cupp M, Rapport CA, Bensadoun A: Secretion and degradation of lipoprotein lipase in cultured adipocytes. J Biol Chem 264: 1767–1774, 1989
Margis R, Pinheiro-Margis M, Silva LCF, Borojevic R: Effects of retinol on proliferation, cell adherence and extracellular matrix synthesis in a liver myofibroblast or lipocyte cell line (GRX). Int J Exp Pathol 73: 125–135, 1992
Burgaya F, Peinado J, Vilaro S, Llobera M, Ramírez l: Lipoprotein lipase activity in neonatal-rat liver cells. Biochem J 259: 159–166, 1989
Vilaro S, Reina M, Ramírez I, Llobera M: Intralipid administration induces a lipoprotein lipase-like activity in the livers of starved rats. Biochem J 236: 273–278, 1986
Blaner WS, Obunike JC, Kurlandsky SB, Al-Haideri M, Piantedosi R, Deckelbaum RJ, Golberg IJ: Lipoprotein lipse hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J Biol Chem 269: 16559–16565, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vicente, C.P., Guaragna, R.M. & Borojevic, R. Lipid metabolism during in vitro induction of the lipocyte phenotype in hepatic stellate cells. Mol Cell Biochem 168, 31–39 (1997). https://doi.org/10.1023/A:1006845808305
Issue Date:
DOI: https://doi.org/10.1023/A:1006845808305