Abstract
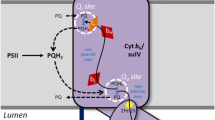
We have found that in isolated spinach thylakoids, plastoquinone-pool (PQ-pool), after its photoreduction, undergoes dark-reoxidation with the half-time of τ1/2 = 43 ± 3 s. To explain the observed rates of PQ-pool reoxidation, a nonenzymatic plastoquinol (PQH2) autoxidation under molecular oxygen and an enzymatic oxidation by the low-potential form of cytochrome b-559 (cyt. b-559LP), as the postulated PQ-oxidase in chlororespiration, were investigated. It was found that the autoxidation rate of PQH2 in organic solvents and liposomes was too low to account for the observed oxidation rate of PQH2 in thylakoids. The rate of cyt. b-559LP autoxidation in isolated Photosystem II was found to be similar (τ1/2 = 26 ± 5 s) to that of the PQ-pool. This suggests that the LP form of cyt. b-559 is probably responsible for the PQ-oxidase activity observed during chlororespiration.
Similar content being viewed by others
References
Ahmad I, Giorgi LB, Barber J, Porter G and Klug DR (1993) Redox potentials of cytochrome b-559 in the D1/D2/cytochrome b-559 reaction centre of Photosystem II. Biochim Biophys Acta 1143: 239–242
Amesz J (1977) Plastoquinone. In: Trebst A and Avron M (eds) Photosynthesis I, Encyclopedia of Plant Physiology, Vol 5, New Series, pp 238–246. Springer-Verlag, Berlin/Heidelberg/New York
Ananyev G, Renger G, Wacker U and Klimov V (1994) The photoproduction of superoxide radicals and the superoxide dismutase activity of photosystem II. The possible involvement of cytochrome b-559. Photosynth Res 41: 327–338
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH and Mullineaux PM (eds) Causes of Photooxidative Stress in Plants and Amelioration of Defense Systems, pp 77–104. CRC Press, Boca Raton, Florida
Barber J and De Las Rivas J (1993) A functional model for the role of cytochrome b-559 in the photoprotection against donor and acceptor side photoinhibition. Proc Natl Acad Sci USA 90: 10942–10946
Barr R and Crane FL (1971) Quinones in algae and higher plants. Methods Enzymol 23A: 372–408
Bendall DS, Davenport HE and Hill R (1971) Cytochrome components in chloroplasts of the higher plants. Meth Enzymol 23A: 327–344
Berthold DA, Babcock GT and Yocum CE (1981) A highly resolved, oxygen-evolving Photosystem II preparation from spinach thylakoid membranes. FEBS Lett 134: 231–234
Buser CA, Diner BA and Brudvig GW (1992a) Reevaluation of the stoichiometry of cytochrome b559 in Photosystem II and thylakoid membranes. Biochemistry 31: 11441–11448
Buser CA, Diner BA and Brudvig GW (1992b) Photooxidation of cytochrome b 559 in oxygen-evolving Photosystem II. Biochemistry 31: 11449–11459
Chapman DJ and Barber J (1986) Analysis of plastoquinone-9 levels in appressed and non-appressed thylakoid membrane regions. Biochim Biophys Acta 850: 170–172
Chapman DJ, Gounaris K and Barber J (1988) Electron-transport properties of the isolated D1-D2-cytochrome b-559 Photosystem II reaction centre. Biochim Biophys Acta 933: 423–431
Goldbeck JH and Kok B (1979) Redox titration of electron acceptor Q and the plastoquinone pool in PS II. Biochim Biophys Acta 547: 347–360
Gounaris K, Chapman DJ and Barber J (1988) Reconstitution of plastoquinone in the D1/D2/cytochrome b-559 Photosystem II reaction centre complex. FEBS Lett 240: 143–147
Graan T and Ort DR (1984) Quantitation of the rapid electron donors to P700, the functional plastoquinone pool, and the ratio of the photosystems in spinach chloroplasts. J Biol Chem 259: 14003–14010
Heimann S and Schreiber U (1996) Characterisation of a H2 O2-oxidisable cytochrome b-559 in intact chloroplasts with a new type of LED array spectrophotometer. Photosynth Res 47: 187–197
Hippler M, Redding K and Rochaix J-D (1998) Chlamydomonas genetics, a tool for the study of bioenergetic pathways. Biochim Biophys Acta 1367: 1–62
Hoefnagel MHN, Atkin OK and Wiskich JT (1988) Interdependence between chloroplasts and mitochondria in the light and the dark.Biochim Biophys Acta 1366: 235–255
Ilan YA Czapski G and Meisel D (1976) The one-electron transfer redox potentials of free radicals. Biochim Biophys Acta 430: 209–224
Izawa S (1977) Inhibitors of electron transport. In: Trebst A and Avron M (eds) Photosynthesis I, Encyclopedia of Plant Physiology, Vol 5, New Series, pp 267–282. Springer-Verlag, Berlin/Heidelberg/New York
Joliot P, Lavegne J and Beal D (1992) Plastoquinone compartmentation in chloroplasts. I. Evidence for domains with different rates of photoreduction. Biochim Biophys Acta 1101: 1–12
Krishtalik LI, Tae G-S, Cherepanov DA and Cramer WA (1993) The redox properties of cytochromes b imposed by the membrane electrostatic environment. Biophys J 65: 184–195
Kruk J (1988) Physicochemical properties of charge-transfer complexes of plastoquinone and α-tocopherol quinone, and their possible role in vivo. Biophys Chem 32: 51–56
Kruk J, Jemio?a-Rzemińska M and Strza?ka K (1997) Plastoquinol and α-tocopherol quinol are more active than ubiquinol and α-tocopherol in inhibition of lipid peroxidation. Chem Phys Lipids 87: 73–80
Kruk J, Strza?ka K and Leblanc RM (1992) Monolayer study of plastoquinones, α-tocopherolquinone, their hydroquinone forms and their interaction with monogalactosyldiacylglycerol. Charge-transfer complexes in a mixed monolayer. Biochim Biophys Acta 1112: 19–26
Kruk J, Strza?ka K and Leblanc RM (1993) Fluorescence properties of plastoquinol, α-tocopherol quinol and ubiquinol. J Photochem Photobiol 19B: 33–38
Landi L, Fiorentini D, Stefanelli C, Pasquali P and Pedulli GF (1990) Inhibition of autoxidation of egg yolk phosphatidylcholine in homogeneous solution and in liposomes by oxidized ubiquinone. Biochim Biophys Acta 1028: 223–228
McCauley SW and Melis A (1986) Quantitation of plastoquinone photoreduction in spinach chloroplasts. Photosynth Res 8: 3–16
McCauley SW, Melis A, Tang GMS and Arnon DI (1987) Protonophores induce plastoquinol oxidation and quench chloroplast fluorescence: evidence for a cyclic, proton-conducting pathway in oxygenic photosynthesis. Proc Natl Acad Sci USA 84: 8424–8428
Nedbal L, Samson G and Whitmarsh J (1992) Redox state of a one-electron component controls the rate of photoinhibition of Photosystem II. Proc Natl Acad Sci USA 89: 7929–7933
Ortega JM, Hervas M and Losada M (1988) Redox and acidbase characterization of cytochrome b-559 in Photosystem II particles. Eur J Biochem 171: 449–455
Ortega JM, Hervas M and Losada M (1989) Isolation and comparison of molecular properties of cytochrome b-559 from both spinach thylakoids and PS II particles. Z Naturforsch 44c: 415–422
Ortega JM, Hervas M and Losada M (1990) Distinctive stability of the reduced and oxidized forms of high-potential cytochrome b-559 in Photosystem II particles. Plant Sci 68: 71–75
Poulson M, Samson G and Whitmarsh J (1995) Evidence that cytochrome b-559 protects Photosystem II against photoinhibition. Biochemistry 34: 10932–10938
Robinson HH and Yocum CF (1980) Cyclic photophosphorylation reactions catalyzed by ferredoxin, methyl viologen and anthraquinone sulfonate. Biochim Biophys Acta 590: 97–106
Schmidt-Mende P and Rumberg B (1968) Zur Plastochinonreduktion bei der Photosynthese. Z Naturforsch 23b: 225–228
Stewart DH and Brudvig GW(1998) Cytochrome b 559 of Photosystem II. Biochim Biophys Acta 1367: 63–87
Stiehl HH and Witt HT (1968) Die kurzzeitigen ultravioletten Differenzspectren bei der Photosynthese. Z Naturforsch 23b: 220–224
Stiehl HH and Witt HT (1969) Quantitative treatment of the function of plastoquinone in photosynthesis. Z Naturforsch 24b: 1588–1598
Whitmarsh J and Cramer WA (1977) Kinetics of the photoreduction of cytochrome b-559 by Photosystem II in chloroplasts. Biochim Biophys Acta 460: 280–289
Whitmarsh J and Cramer WA (1978) A pathway for the reduction of cytochrome b-559 by Photosystem II in chloroplasts. Biochim Biophys Acta 501: 83–93
Whitmarsh J, Samson G and Poulson M (1994) Photoprotection in Photosystem II - the role of cytochrome b-559. In: Baker NR and Bowyer JR (eds) Photoinhibition of Photosynthesis, pp 75–93. Bios Scientific Publishers, Oxford, UK
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kruk, J., Strzałka, K. Dark reoxidation of the plastoquinone-pool is mediated by the low-potential form of cytochrome b-559 in spinach thylakoids. Photosynthesis Research 62, 273–279 (1999). https://doi.org/10.1023/A:1006374319191
Issue Date:
DOI: https://doi.org/10.1023/A:1006374319191