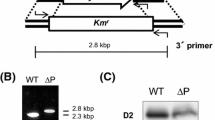
Abstract
The turnover of the D1 and D2 proteins of Photosystem II (PSII) has been investigated by pulse-chase radiolabeling in several strains of the cyanobacterium Synechocystis PCC 6803 containing different types and levels of the psbA transcript. Strains lacking psbA1 and psbA3 gene and containing high levels of the psbA2 transcript showed the selective synthesis of D1 whose degradation could be slowed down by the protein synthesis inhibitor lincomycin. In contrast, in strains containing just the psbA3 gene, the intensity of the D1 protein labeling was lower and labeling of the D2 and CP43 proteins was stimulated in comparison to the psbA2-containing strains. In addition, the rate and selectivity of the D1 degradation and its dependence on the presence of lincomycin was proportional to the level of the psbA3 transcript in the particular strain. Consequently, there was parallel, lincomycin-independent and slowed-down breakdown of the D1 and D2 proteins in strains with the lowest level of psbA3 transcript. These results are discussed in terms of a model in which the rate of D1 and D2 degradation in cyanobacteria is affected not only by the rate of PSII photodamage, but also by the availability of newly synthesized D1 protein. Moreover, the comparison of the non-oxygen-evolving D1 mutants D170A** and Y161F*** differing by the presence of tyrosine Z has indicated a minor role of the oxidized form of this secondary PSII electron donor in the donor side mechanism of D1 and D2 protein breakdown.
Similar content being viewed by others
References
Aro, E.-M., Virgin, I. and Andersson, B. 1993. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143: 113–134.
Barber, J. 1994. Molecular basis of the vulnerability of photosystem II to damage by light. Aust. J. Plant Physiol. 22: 201–208.
Blubaugh, D.J., Atamian, M., Babcock, G.T., Golbeck, J.H. and Cheniae, G.M. 1991. Photoinhibition of hydroxylamineextracted photosystem II membranes: identification of the sites of photodamage. Biochemistry 30: 7586–7597.
Chen, G.-X., Blubaugh, D.J., Homann, P.H., Golbeck, J.H. and Cheniae, G.M. 1995. Superoxide contributes to the rapid inactivation of specific secondary donors of the photosystem II reaction center during photodamage of manganese-depleted photosystem II membranes. Biochemistry 34: 2317–2332.
Chu, H.-A., Nguyen, A.P. and Debus, R.J. 1994a. Site-directed Photosystem II mutants with perturbed oxygen-evolving properties. 1. Instability or inefficient assembly of the manganese cluster in vivo. Biochemistry 33: 6137–6149.
Chu, H.-A., Nguyen, A.P. and Debus, R.J. 1994b. Site-directed Photosystem II mutants with perturbed oxygen-evolving properties. 2. Increased binding or photooxidation of manganese in the absence of the extrinsic 33-kDa polypeptide in vivo. Biochemistry 33: 6150–6157.
Dalla Chiesa, M., Friso, G., Deak, Z., Vass, I., Barber, J. and Nixon, P.J. 1997. Reduced turnover of the D1 polypeptide and photoactivation of electron transfer in novel herbicide resistant mutants of Synechocystis sp. PCC 6803. Eur. J. Biochem. 248: 731–740.
Debus, R.J., Barry, B.A., Sithole, I., Babcock, G.T. and McIntosh, L. 1988. Directed mutagenesis indicates that the donor to P680C in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry 27: 9071–9074.
De Las Rivas, J., Telfer, A. and Barber, J. 1993. Two coupled β-carotene molecules protect P680 from photodamage in isolated photosystem II reaction centres. Biochim. Biophys. Acta. 1142: 155–164.
Erickson, J.M. and Rochaix, J.D. 1992. The molecular biology of photosystem II. In: J. Barber (Ed.) The Photosystems: Structure, Function and Molecular Biology, Vol. 11, Elsevier, Amsterdam, pp. 101–177.
Gong, H. 1994. Light-dependent degradation of the photosystem II D1 protein is retarded by inhibitors of chloroplast transcription and translation: possible involvement of a chloroplast-encoded proteinase. Biochim. Biophys. Acta 1186: 143–152.
Hideg, E., Spetea, C. and Vass, I. 1994. Singlet oxygen and free radical production during acceptor-and donor-side-induced photoinhibition. Studies with spin trapping EPR spectroscopy. Biochim. Biophys. Acta 1186: 143–152.
Jansen, M.A.K., Depka, B., Trebst, A. and Edelman, M. 1993. Engagement of specific sites in the plastoquinone niche regulates degradation of the D1 protein in photosystem II. J. Biol. Chem. 268: 21246–21252.
Keren, N., Berg, A., van Kan, P.J.M., Levanon, H. and Ohad, L. 1997. Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc. Natl. Acad. Sci. USA 94: 1579–1584.
Komenda, J. and Barber, J. 1995. Comparison of psbO and psbH deletion mutants of Synechocystis PCC 6803 indicate that degradation of D1 protein is regulated by the QB site and is dependent on protein synthesis. Biochemistry 34: 9625–9631.
Komenda, J. and Masojídek, J. 1995. Structural changes of Photosystem II complex induced by high irradiance in cyanobacterial cells. Eur. J. Biochem. 233: 677–682.
Komenda, J., Koblížek, M. and Masojídek, J. 1999. The regulatory role of Photosystem II photoinactivation and de novo protein synthesis in the degradation and exchange of two forms of the D1 protein in the cyanobacterium Synechococcus PCC 7942. J. Photochem. Photobiol. 48: 114–119.
Krieger, A.A., Rutherford, W., Vass, I. and Hideg, E. 1998. Relationship between activity, D1 loss, and Mn binding in photoinhibition of photosystem II. Biochemistry 37: 16262–16269.
Kullander, C., Fredriksson, P.O., Sayre, R.T., Minagawa, J., Crofts, A.R. and Styring, S. 1995. Electron donation from exogenous donors to photosystem II studied in Chlamydomonas reinhardtii mutants. In: P. Mathis (Ed.) Photosynthesis: From Light to Biosphere, Vol. II, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 321–324.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Macpherson, A.N., Telfer, A., Barber, J. and Truscott, T.G. 1993. Direct detection of singlet oxygen from isolated photosystem II reaction centres. Biochim Biophys. Acta 1143: 301–309.
Máté Z, Sass, L., Szekeres, M., Vass, I. and Nagy, F. 1998. UV-Binduced differential transcription of psbA genes encoding the D1 protein of photosystem II in the cyanobacterium Synechocystis 6803. J. Biol. Chem. 273: 17439–17444.
Mattoo, A.K., Hoffman-Falk, H., Marder, J.B. and Edelman, M. 1984. Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc. Natl. Acad. Sci. USA 81: 1380–1384.
Mattoo, A., Marder, J.B. and Edelman, M. 1989. Dynamics of the photosystem II reaction center. Cell 56: 241–246 (1989).
Metz, J.G., Nixon, P.J. Rögner, M., Brudvig, G.W. and Diner, B.A. 1989. Directed alteration of the D1 polypeptide of photosystem II: evidence that tyrosine-161 is the redox component, Z, connecting the oxygen evolving complex to the primary electron donor, P680. Biochemistry 28: 6960–6969.
Mishra, N.P. and Ghanotakis, D.F. 1994. Exposure of a photosystem II to chemically generated singlet oxygen results in D1 fragments similar to ones observed during aerobic photoinhibition. Biochim. Biophys. Acta 1187: 296–300.
Miyao, M. 1994. Involvement of active oxygen species in degradation of the D1 protein under strong illumination in isolated subcomplexes of photosystem II. Biochemistry 33: 9722–9730.
Miyao, M., Ikeuchi, M., Yamamoto, N. and Ono, T. 1995. Specific degradation of the D1 protein of photosystem II by treatment with hydrogen peroxide in darkness: implication for the mechanism of degradation of the D1 protein under illumination. Biochemistry 34: 10019–10026.
Mohamed, A. and Jansson, Ch. 1989. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 13: 693–700.
Mohamed, A., Eriksson, J., Osiewacz, H.D. and Jansson, Ch. 1993. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol. Gen. Genet. 238: 161–168.
Mulo, P., Tyystjärvi, T., Tyystjärvi, E., Mäenpää, P. and Aro, E.-M. 1997. Mutagenesis of the D-E loop of photosystem II reaction centre protein D1. Function and assembly of photosystem II. Plant Mol. Biol. 33: 1059–1071.
Nanba, O. and Satoh, K. 1987. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc. Natl. Acad. Sci. USA 84: 109–112.
Nixon, P.J. and Diner, B.A. 1992. Aspartate 170 of the photosystem II reaction center polypeptide D1 is involved in the assembly of the oxygen-evolving manganese cluster. Biochemistry 31: 942–948.
Nixon, P.J., Metz, J.G., Rögner, M. and Diner, B. 1990. A Synechocystis PCC 6803 psbA deletion mutant and its transformation with a psbA gene from a higher plant. In: M. Baltscheffsky (Ed.) Current Research in Photosynthesis, Vol. I, Kluwer Academic Publishers, Dordrecht, Netherlands, pp. 471–474.
Nixon, P.J., Trost, J.T. and Diner, B.A. 1992. Role of the carboxy terminus of polypeptide D1 in the assembly of a functional water oxidizing manganese cluster in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: assembly requires a free carboxyl group at C-terminal position 344. Biochemistry 31: 10859–10871.
Nixon, P.J., Komenda, J., Barber, J., Deak, Z., Vass, I. and Diner, B.A. 1995. Deletion of the PEST-like region of Photosystem-2 modifies the Q(B)-binding pocket but does not prevent rapid turnover of D1. J. Biol. Chem. 270: 14919–14927.
Ohad, I., Keren, A., Zer, H., Gong, H., Mor, T.S., Gal, A., Gal, S. and Domovich, Y. 1994. Light-induced degradation of the photosystem II reaction centre D1 protein in vivo: an integrative approach. In: N.R. Baker and J.R. Bowyer (Eds.) Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field, BIOS Scientific Publishers, Oxford, UK, pp. 161–177.
Pakrasi, H.B., Williams, J.G.K. and Arntzen, C.J. 1988. Targeted mutagenesis of the psbE and psbF blocks photosynthetic electron transport: evidence for a functional role of cytochrome b-559 in photosystem II. EMBO J. 7: 325–332.
Tyystjärvi, T., Mulo, P., Mäenpää, P. and Aro, E.-M. 1996. D1 polypeptide degradation may regulate psbA gene expression at transcriptional and translational levels in Synechocystis sp. PCC 6803. Photosyn. Res. 47: 111–120.
Vass, I., Styring, S., Hundal, T., Koivuniemi, A., Aro, E.-M. and Andersson, B. 1992. Reversible and irreversible intermediates during photoinhibition of Photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc. Natl. Acad. Sci. USA 89: 1048–1412.
Wellburn, A.R. and Lichtenthaler, H.K. 1984. Formulae and programme to determine total carotenoids and chlorophyll a and b of leaf extracts in different solvents. In: C. Sybesma (Ed.) dvances in Photosynthesis Research, Vol. II, Martinus Nijhoff, Dordrecht, Netherlands, pp. 10–12.
Williams, J.G.K. 1988. Construction of specific mutations in PSII photosynthetic reaction center by genetic engineering methods in Synechocystis 6803. Meth. Enzymol. 167: 766–778.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Komenda, J., Hassan, H.A., Diner, B.A. et al. Degradation of the Photosystem II D1 and D2 proteins in different strains of the cyanobacterium Synechocystis PCC 6803 varying with respect to the type and level of psbA transcript. Plant Mol Biol 42, 635–645 (2000). https://doi.org/10.1023/A:1006305308196
Issue Date:
DOI: https://doi.org/10.1023/A:1006305308196