Abstract
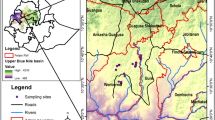
Littoral zones of small water bodies are spatially heterogeneous habitats, harbouring diverse biotic communities. Despite this apparent heterogeneity, many studies have stressed the importance of water chemistry in determining the structure of littoral macroinvertebrate assemblages. The purpose of this study was to consider the relative importance of several spatial and water chemistry variables in explaining the patterns in the structure of macroinvertebrate assemblages in 21 lentic water bodies in northeastern Finland. Water bodies were selected to represent various habitat conditions ranging from small permanent bog ponds to small forest lakes. According to canonical correspondence analysis (CCA), the most important environmental factors related to assemblage composition were water body area, moss cover, total nitrogen and water hardness. In general, species composition in small bog ponds tended to differ from that in larger lakes with forested shoreline. Total species richness was best explained by a composite variable (PCA) describing physical habitat heterogeneity, species richness being lowest in small bog lakes with simple bottom structure and low amount of aquatic plants. Species numbers in dominant functional feeding groups were related to different environmental factors. Shredder species richness was best explained by a regression model incorporating total nitrogen and the amount of organic matter, both of which were negatively related to the number of shredder species. The number of gatherer species increased with mean substratum particle size. Scraper species richness was negatively affected by the abundance of detritus and positively affected by depth, and a model including both variables explained most of the variation. Variation in the number of predatory species was best explained by a regression model including moss cover and lake area.
Similar content being viewed by others
References
Aho, J. M., 1978. Freshwater snail populations and the equilibrium theory of island biogeography. II. Relative importance of chemical and spatial variables. Ann. Zool. Fennici 15: 155–164.
Ahti, T., L. Hämet-Ahti & J. Jalas, 1968. Vegetation zones and their sections in northern Europe. Ann. bot. fenn. 5: 169–211.
Allan, J. D., 1995. Stream Ecology. Structure and Function of Running Waters. Chapman and Hall, London: 400 pp.
Batzer, D. P. & S. A. Wissinger, 1996. Ecology of insect communities on nontidal wetlands. Ann. Rev. Ent. 41: 75–100.
Begon, M., J. L. Harper & C. R. Townsend, 1996. Ecology. 3rd edn. Blackwell, Oxford: 1068 pp.
Bendell, B. E. & D. K. McNicol, 1987. Fish predation, lake acidity and the composition of aquatic insect assemblages. Hydrobiologia 150: 193–202.
Bendell, B. E. & D. K. McNicol, 1995. Lake acidity, fish predation and the distribution and abundance of some littoral insects. Hydrobiologia 302: 133–145.
Bennet, D. V. & F. A. Streams, 1986. Effects of vegetation on Notonecta (Hemiptera) distribution in ponds with and without fish. Oikos 46: 62–69.
Brodersen, K. P., P. C. Dall & C. Lindegaard, 1998. The invertebrate fauna in the upper stony littoral of Danish lakes: macroinvertebrates as trophic indicators. Freshwat. Biol. 39: 577–592.
Brown, C. L., P. P. Poe, J. R. P. French & D. W. Schlosser, 1988. Relationships of phytomacrofauna to surface area in naturally occurring macrophyte stands. J. n. am. Benthol. Soc. 7: 129–139.
Brönmark, C., 1985. Freshwater snail diversity: effects of pond area, habitat heterogeneity and isolation. Oecologia 67: 127–131.
Carpenter, S. R. & D. M. Lodge, 1986. Effects of submerged macrophytes on ecosystem processes. Aquat. Bot. 26: 341–370.
Connor, E. F. & E. D. McCoy, 1979. The statistics and biology of the species area relationship. Am. Nat. 113: 791–833.
Cummins, K. W., 1973. Trophic relationships of aquatic insects. Ann. Rev. Ent. 18: 183–206.
Diehl, S., 1992. Fish predation and benthic community structure: the role of omnivory and habitat complexity. Ecology 73: 1646–1661.
Duarte, C. & J. Kalff, 1986. Littoral slope as a predictor of maximum biomass of submerged macrophyte communities. Limnol. Oceanogr. 31: 1072–1080.
Eadie, J. M. & A. Keast, 1984. Resource heterogeneity and fish species diversity in lakes. Can. J. Zool. 62: 1689–1695.
Edington, J. M. & A. G. Hildrew, 1995. A revised key to the caseless caddis larvae of the British Isles. Freshwat. Biol. Ass. Scient. Publ. 53: 1–138.
Elliott, J. M., U. H. Humbesch & T. T. Macan, 1988. Larvae of the British Ephemeroptera. A key with ecological notes. Freshwat. Biol. Ass. Scient. Publ. 49: 1–151.
Engblom, E., P. E. Lingdell & A. N. Nilsson, 1990. Sveriges bäckbaggar (Coleoptera, Elmidae)-artbestämning, habitatval och värde som miljöindikatorer. Entomol. Tidskr. 11: 105–121 (in Swedish).
France, R., 1990. Epiphytic zoobenthos density and biomass within low alkalinity, oligotrophic lakes on the Canadian Shield. Arch. Hydrobiol. 118: 477–499.
France, R. L., 1995. Macroinvertebrate standing crop in littoral regions of allochthonous detritus accumulation: implications for forest management. Biol. Cons. 71: 35–39.
Friday, L. E., 1987. The diversity of macroinvertebrate and macrophyte communities in ponds. Freshwat. Biol. 18: 87–104.
Fryer, G., 1985. Crustacean diversity in relation to the size of water bodies: some facts and problems. Freshwat. Biol. 15: 347–361.
Gauch, H. G., 1982. Multivariate Analysis in Community Ecology. Cambridge University Press, Cambridge: 298 pp.
Gilinsky, E., 1984. The role of fish predation and spatial heterogeneity in determining benthic community structure. Ecology 65: 455–468.
Hanlon, R. D., 1981. Allochtonous plant litter as a source of organic material in an oligotrophic lake (Llyn Frongoch). Hydrobiologia 80: 257–261.
Henrikson, B.-I., 1993. Sphagnum mosses as a microhabitat for invertebrates in acidified lakes and the color adaptation and substrate preference in Leucorrhinia dubia (Odonata, Anisoptera). Ecography 16: 143–153.
Hildrew, A. G. & C. R. Townsend, 1987. Organization in freshwater benthic communities. In Gee, J. H. R. & P. S. Giller (eds), Organization of Communities Past and Present. Blackwell, Oxford: 347–371.
Hill, J. L., P. J. Curran & G. M. Foody, 1994. The effect of sampling on the species-area curve. Glob. Ecol. Biodiv. Lett. 4: 97–106.
Hoffman, R. L., W. J. Liss, G. L. Larson, E. K. Deimling & G. A. Lomnicky, 1996. Distribution of nearshore macroinvertebrates in lakes of the northern Cascade Mountains, Washington, U.S.A. Arch. Hydrobiol. 136: 363–389.
Huston, M. L., 1994. Biological Diversity. The Coexistence of Species on Changing Landscapes. Cambridge University Press, Cambridge: 682 pp.
Jeffries, M., 1989. Measuring Talling's 'element of chance' in pond populations. Freshwat. Biol. 21: 383–393.
Jeffries, M., 1991. The ecology and conservation value of forestry ponds in Scotland, United Kingdom. Biol. Cons. 58: 191–211.
Jones, P. D. & Momot, W. T., 1981. Crayfish productivity, allocthony, and basin morphometry. Can. J. Fish. aquat. Sci. 38: 175–183.
Larson, D. L., 1985. Structure in temperate predaceous diving beetle communities (Coleoptera, Dytiscidae. Holarct. Ecol. 8: 18–32.
MacArthur, R. J. & E. O. Wilson, 1967. The Theory of Island Biogeography. Princeton University Press, New Jersey: 216 pp.
McCune, B., 1997. Influence of noisy environmental data on canonical correspondence analysis. Ecology 78: 2617–2623.
McLachlan, A. J. & S. M. McLachlan, 1975. The physical environment and bottom fauna of a bog lake. Hydrobiologia 76: 198–217.
Merritt, R. W. & K. W. Cummins (eds), 1984. An Introduction to the Aquatic Insects of North America. 2nd edn. Kendall/Hunt, Dubuque: 722 pp.
Minshall, G. W., 1984. Aquatic insect-substratum relationships. In Resh, V. H. & D. M. Rosenberg (eds), The Ecology of Aquatic Insects. Praeger, New York: 358–400.
Nilsson, A. N., J. Elmberg & K. Sjöberg, 1994. Abundance and species richness patterns of diving beetles (Coleoptera, Dytiscidae) in Swedish lakes. J. Biogeography 21: 197–206.
Nilsson, A. N. & H. Söderberg, 1996. Abundance and distribution patterns of diving beetles (Coleoptera, Dytiscidae) from exposed and protected sites in 98 northern Swedish lakes. Hydrobiologia 321: 83–88.
Okland, J., 1990. Lakes and Snails. Universal Book Services/W Backhyus, Oegstgeest.
Palmer, M. W., 1993. Putting things in even better order: the advantages of canonical correspondence analysis. Ecology 74: 2215–2230.
Pennak, R. W., 1978. Freshwater Invertebrates of the United States. John Wiley & Johns, New York: 803 pp.
Rahel, F., 1984. Factors structuring fish assemblages along a bog lake successional gradient. Ecology 65: 1276–1289.
Ranta, E., 1985. Communities of water beetles in different kinds of water in Finland. Proc Acad. nat. Sci. Philad. 137: 33–45.
Rasmussen, J. B., 1988. Littoral zoobenthic biomass in lakes, and its relationship to physical, chemical and trophic factors. Can. J. Fish. aquat. Sci. 45: 1436–1447.
Rasmussen, J. B. & J. Kalff, 1987. Empirical models for zoobenthic biomass in lakes. Can. J. Fish. aquat. Sci. 44: 990–1001.
Robinson, C. L. K. & W. M. Tonn, 1989. Influence of environmental factors and piscivory in structuring fish assemblages of small Alberta lakes. Can. J. Fish. aquat. Sci. 46: 81–89.
Rodriquez, M. A. & W. M. Lewis, 1997. Structure of fish assemblages along environmental gradients in floodplain lakes of the Orinoco River. Ecol. Monogr. 67: 109–128.
Rosenzweig, M. J., 1995. Species Diversity in Space and Time. Cambridge University Press, Cambridge: 436 pp.
Sokal, R. & J. Rolf, 1994. Biometry. The Principles and Practice of Statistics in Biological Research. Freeman, New York: 887 pp.
ter Braak, C. J. F., 1991. Program CANOCO, version 3.12. Agricultural Mathematics Group DLO, Wageningen.
ter Braak, C. J. F., 1995. Ordination. In Jongman, R. H. G., C. J. F. ter Braak & O. F. Van Tongeren (eds), Data Analysis in Community and Landscape Ecology. Cambridge University Press, Cambridge: 91–173.
Tonn, W. M. & J. J. Magnuson, 1982. Patterns in the species composition and richness of fish assemblages in northern Wisconsin lakes. Ecology 63: 1149–1166.
Wallace, H. B. & J. R. Webster, 1996. The role of macroinvertebrates in stream ecosystem function. Ann. Rev. Ent. 41: 115–139.
Webster, J. R. & E. F. Benfield, 1986. Vascular plant breakdown in freshwater ecosystems. Ann. Rev. Ecol. Syst. 17: 567–594.
Wellborn, G. A., D. K. Skelly & E. W. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Ann. Rev. Ecol. Syst. 27: 337–363.
Wetzel, R. G., 1983. Limnology. 2nd edn. Saunders, New York: 767 pp.
Wissmar, R. C., 1991. Forest detritus and cycling of nitrogen in a mountain lake. Can. J. Forest Res. 21: 990–998.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heino, J. Lentic macroinvertebrate assemblage structure along gradients in spatial heterogeneity, habitat size and water chemistry. Hydrobiologia 418, 229–242 (2000). https://doi.org/10.1023/A:1003969217686
Issue Date:
DOI: https://doi.org/10.1023/A:1003969217686