Abstract
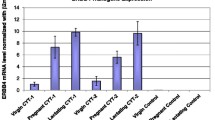
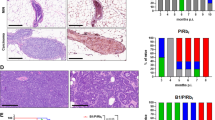
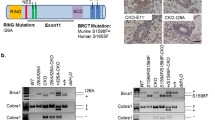
To address the hypothesis that certain disease-associated mutants of the breast-ovarian cancer susceptibility gene BRCA1 have biological activity in vivo, we have expressed a truncated Brca1 protein (trBrca1) in cell-lines and in the mammary gland of transgenic mice. Immunofluorescent analysis of transfected cell-lines indicates that trBRCA1 is a stable protein and that it is localized in the cell cytoplasm. Functional analysis of these cell-lines indicates that expression of trBRCA1 confers an increased radiosensitivity phenotype on mammary epithelial cells, consistent with abrogation of the BRCA1 pathway. MMTV-trBrca1 transgenic mice from two independent lines displayed a delay in lactational mammary gland development, as demonstrated by altered histological profiles of lobuloalveolar structures. Cellular and molecular analyses indicate that this phenotype results from a defect in differentiation, rather than altered rates of proliferation or apoptosis. The results presented in this paper are consistent with trBrca1 possessing dominant-negative activity and playing an important role in regulating normal mammary development. They may also have implications for germline carriers of BRCA1 mutations.
Similar content being viewed by others
References
Amundadottir LT, Merlino G and Dickson RB (1996) Transgenic mouse models of breast cancer. Breast Cancer Res Treat 39: 119–135.
Brugarolas J and Jacks T (1997) Double indemnity: p53, BRCA and cancer. p53 mutation partially rescues developmental ar-rest in Brca1 and Brca2 null mice, suggesting a role for fa-milial breast cancer genes in DNA damage repair. Nat Med 3: 721–722.
Connor F, Bertwistle D, Mee PJ, Ross GM, Swift S, Grigorieva E et al. (1997) Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat Genet 17: 423–430.
Deng CX and Brodie SG (2000) Roles of BRCA1 and its interacting proteins. Bioessays 22: 728–737.
Englert C, Vidal M, Maheswaran S, Ge Y, Ezzell RM, Isselbacher KJ et al. (1995) Truncated WT1 mutants alter the subnuclear localization of the wild-type protein. Proc Natl Acad Sci USA 92: 11960–11964.
Fan S, Yuan R-Q, Ma YX, Meng Q, Goldberg ID and Rosen EM (2001) Mutant BRCA1 genes antagonize phenotype of wild-type BRCA1. Oncogene 20: 8215–8235.
Foray N, Randrianarison V, Marot D, Perricaudet M, Lenoir G and Feunteun J (1999) Gamma-rays-induced death of human cells carrying mutations of BRCA1 or BRCA2. Oncogene 18: 7334-7342.
Gudas JM, Nguyen H, Li T and Cowan KH (1995) Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res 55: 4561–4565.
Haber DA, Timmers HT, Pelletier J, Sharp PA and Housman DE (1992) A dominant mutation in the Wilms tumor gene WT1 cooperates with the viral oncogene E1A in transformation of primary kidney cells. Proc Natl Acad Sci USA 89: 6010-6014.
Harvey M, Vogel H, Morris D, Bradley A, Bernstein A and Done-hower LA (1995) A mutant p53 transgene accelerates tumour development in heterozygous but not nullizygous p53-deficient mice. Nat Genet 9: 305–311.
Jackson D, Bresnick J, Rosewell I, Crafton T, Poulsom R, Stamp G et al. (1997) Fibroblast growth factor receptor signalling has a role in lobuloalveolar development of the mammary gland. J Cell Sci 110: 1261–1268.
Jernstrom H, Johannsson O, Borg A, Ivarsson H and Olsson H (1998) BRCA1-positive patients are small for gestational age compared with their unaffected relatives. Eur J Cancer 34: 368–371.
Kern SE, Pietenpol JA, Thiagalingam S, Seymour A, Kinzler KW and Vogelstein B (1992) Oncogenic forms of p53 inhibit p53 regulated gene expression. Science 256: 827–830.
Kinzler KW and Vogelstein B (1997) Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 386: 761–763.
Lane TF, Deng C, Elson A, Lyu MS, Kozak CA and Leder P (1995) Expression of Brca1 is associated with terminal differentiation of ectodermally and mesodermally derived tissues in mice. Genes Dev 9: 2712–2722.
Larson JS, Tonkinson JL and Lai MT (1997) A BRCA1 mutant al-ters G2-M cell cycle control in human mammary epithelial cells. Cancer Res 57: 3351–3355.
Lindeman GJ, Wittlin S, Lada H, Naylor MJ, Santamaria M, Zhang JG et al. (2001) SOCS1 deficiency results in accelerated mammary gland development and rescues lactation in prolactin receptor-deficient mice. Genes Dev 15: 1631–1636.
Marks JR, Huper G, Vaughn JP, Davis PL, Norris J, McDonnell DP et al. (1997) BRCA1 expression is not directly responsive to estrogen. Oncogene 14: 115–121.
Marquis ST, Rajan JV, Wynshaw-Boris A, Xu J, Yin GY, Abel KJ et al. (1995) The developmental pattern of Brca1 expression implies a role in differentiation of the breast and other tissues. Nat Genet 11: 17–26.
Morgan SE, Lovly C, Pandita TK, Shiloh Y and Kastan MB (1997) Fragments of ATM which have dominant-negative or complementing activity. Mol Cell Bio l17: 2020–2029.
Nicolaides NC, Littman SJ, Modrich P, Kinzler KW and Vogelstein B (1998) A naturally occurring hPMS2 mutation can confer a dominant negative mutator phenotype. Mol Cell Biol 18: 1635-1641.
Pullan SE and Streuli CH (1996) The mammary gland epithelial cells. In: Harris A (ed.), Epithelial Cell Culture. (pp 97–121) Cambridge University Press.
Robinson GW, McKnight RA, Smith GH and Hennighausen L (1995) Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 121: 2079–2090.
Stewart T, Pattengale PK and Leder P (1984) Spontaneous mam-mary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell 38: 627–637.
Thangaraju M, Kaufmann SH and Couch FJ (2000) BRCA1 facilitates stress-induced apoptosis in breast and ovarian cancer cell lines. J BiolChem 275: 33487–33496.
Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T et al. (1999) Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet 22: 37–43.
Zhang H, Somasundaram K, Peng Y, Tian H, Bi D, Weber BL et al. (1998) BRCA1 physically associates with p53 and stimulates its transcriptional activity. Oncogene 16: 1713–1721.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, M.A., Nicolai, H., Howe, K. et al. Expression of a Truncated Brca1 Protein Delays Lactational Mammary Development in Transgenic Mice. Transgenic Res 11, 467–478 (2002). https://doi.org/10.1023/A:1020348025139
Issue Date:
DOI: https://doi.org/10.1023/A:1020348025139