Abstract
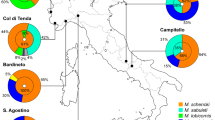
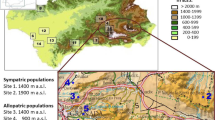
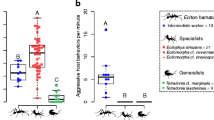
The ant social parasite, Maculinea rebeli shows high levels of host specificity at a regional scale. While 68–88% of caterpillars in the field are adopted by nonhost Myrmica ants, 95–100% of the butterflies emerge from the natural host M. schencki the following year. While retrieval of preadoption caterpillars is specific to the genus Myrmica, it does not explain differential survival with different Myrmica species. We present survival data with host and nonhost Myrmica species suggesting that, with nonhosts (M. sabuleti and M. rubra), survival depends on the physiological state of the colony. We also compared the similarities of the epicuticular surface hydrocarbon signatures of caterpillars that were reared by host and nonhost Myrmica for 3 weeks with those from tending workers. Counterintuitively, the hydrocarbons of postadoption caterpillars were more similar (78%, 73%) to the ant colony profiles of the nonhost species than were caterpillars reared in colonies of M. schencki (42% similarity). However, caterpillars from M. schencki nests that were then isolated for 4 additional days showed unchanged chemical profiles, whereas the similarities of those from nonhost colonies fell to 52 and 56%, respectively. Six compounds, presumably newly synthesized, were detected on the isolated caterpillars that could not have been acquired from M. sabuleti and M. rubra (nor occurred on preadoption caterpillars), five of which were found on the natural host M. schencki. These new compounds may relate to the high rank the caterpillars attain within the hierarchy of M. schencki societies. The same compounds would identify the caterpillars as intruders in non-schencki colonies, where their synthesis appeared to be largely suppressed. The ability to synthesize or suppress additional compounds once adopted explains the pattern of mortalities found among fully integrated caterpillars in Myrmica colonies of different species and physiological states.
Similar content being viewed by others
References
Akino,T., Knapp,J. J., Thomas,J. A., and Elmes,G. W. 1999. Chemical mimicry and host specificity in the butterfly Maculinea rebeli,a social parasite of Myrmica ant colonies. Proc. R. Soc. London, Ser. B-Biol. Sci. 266:1419-1426.
Als,T. D., Nash,D. R., and Boomsma,J. J. 2001. Adoption of parasitic Maculinea alcon caterpillars (Lepidoptera: Lycaenidae) by three Myrmica ant species. Ani. Behav. 62:99-106.
Als,T. D., Nash,D. R., and Boomsma,J. J. 2002. Geographical variation in host-ant specificity of the parasitic butterfly. Maculinea alcon in Denmark. Ecol. Entomol. 27:403-414.
Carr,M. R. 1996. Primer User Manual (Plymouth routines in multivariate ecological research), Version 4.0b. Plymouth Marine Laboratory, Plymouth, UK.
Clarke,K. R. 1993. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 18:117-143.
Dani,F. R., Jones,G. R., Destri,S., Spencer,S. H., and Turillazzi,S. 2001. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Ani. Behav. 62:165-171.
Dettner,K. and Liepert,C. 1994. Chemical mimicry and camouflage. Annu. Rev. Entomol. 39:129-154.
DeVries,P. J., Cocroft,R. B., and Thomas,J. 1993. Comparison of acoustical signals in Maculinea butterfly caterpillars and their obligate host Myrmica ants. Biol. J. Linnean Soc. 49:229-238.
Elmes,G. W., Akino,T., Thomas,J. A., Clarke,R. T., and Knapp,J. J. 2002. Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525-535.
Elmes,G. W., Barr,B., Thomas,J. A., and Clarke,R. T. 1999. Extreme host specificity by Microdon mutabilis (Diptera: Syrphidae), a social parasite of ants. Proc. R. Soc. London Ser. B-Biol. Sci. 266:447-453.
Elmes,G. W., Thomas,J. A., Munguira,M. L., and Fiedler,K. 2001. Larvae of lycaenid butterflies that parasitize ant colonies provide exceptions to normal insect growth rules. Biol. J. Linnean Soc. 73:259-278.
Elmes,G. W., Thomas,J. A., and Wardlaw,J. C. 1991b. Larvae of Maculinea rebeli,a large-blue butterfly, and their Myrmica host ants–Wild adoption and behavior in ant-nests. J. Zool. 223:447-460.
Elmes,G. W., Wardlaw,J. C., Schönrogge,K., Thomas,J. A., and Clarke,R. T. 2004. Food stress causes differential survival of socially parasitic caterpillars of Maculinea rebeli integrated in colonies of host and non-host Myrmica species. Entomol. Exp. Appl.
Elmes,G. W., Wardlaw,J. C., and Thomas,J. A. 1991a. Larvae of Maculinea rebeli,a large-blue butterfly and their Myrmica,host ants–Patterns of caterpillar growth and survival. J. Zool. 224:79-92.
Fiedler,K. 1998. Lycaenid–ant interactions of the Maculinea type: Tracing their historical roots in a comparative framework. J. Insect Conserv. 2:3-14.
Fiedler,K. 1990. New information on the biology of Maculinea nausithous and M. teleius (Lepidoptera: Lycaenidae). Nota Lepid 12:246-256.
Fiedler,K. 1991. Systematic, evolutionary, and ecological implications of Myrmecophily within Lycaenidae (Insecta: Lepidoptera: Papilionidae). Zoologisches Forschungsinstitut und Museum Alexander König, Bonn.
Hölldobler,B. and Wilson,E. O. 1990. The Ants. Springer Verlag, Berlin.
Lenoir,A., D'Ettorre,P., Errard,C., and Hefetz,A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46:573-599.
Pierce,N. E., Braby,M. F., Heath,A., Lohman,D. J., Mathew,J., Rand,D. B., and Travassos,M. A. 2002. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera). Annu. Rev. Entomol. 47:733-771.
Pierce,N. E., Kitching,R. L., Buckley,R. C., Taylor,M. F. J., and Benbow,K. F. 1987. The costs and benefits of cooperation Between the Australian Lycaenid Butterfly, Jalmenus evagoras,and its attendant ants. Behav. Ecol. Sociobiol. 21:37-248.
Schönrogge,K., Barr,B., Wardlaw,J. C., Napper,E., Gardner,M. G., Breen,J., Elmes,G. W., and Thomas,J. A. 2002. When rare species become endangered: Cryptic speciation in myrmecophilous hoverflies. Biol. J. Linnean Soc. 75:291-300.
Schönrogge,K., Wardlaw,J. C., Thomas,J. A., and Elmes,G. W. 2000. Polymorphic growth rates in myrmecophilous insects. Proc. R. Soc. London Ser. B-Biol. Sci. 267:771-777.
Steiner,F. M., Sielezniew,M., Schlick-Steiner,B. C., Höttinger,H., Stankiewicz,A., and Gornicki,A. 2003. Host specificity revisited: New data on Myrmica host ants of the vulnerable lycaenid butterfly Maculinea rebeli. J. Insect Conserv. 7:1-6.
Thomas,J. A. 2002. Larval niche selection and evening exposure enhance adoption of a predacious social parasite, Maculinea arion (large blue butterfly), by Myrmica ants. Oecologia 122:531-537.
Thomas,J. A. and Wardlaw,J. C. 1992. The capacity of a Myrmica ant nest to support a predacious species of Maculinea butterfly. Oecologia 91:101-109.
Thomas,J. A. and Elmes,G. W. 1998. Higher productivity at the cost of increased host-specificity when Maculinea butterfly larvae exploit ant colonies through trophallaxis rather than by predation. Ecol. Entomol. 23:57-464.
Thomas,J. A., Elmes,G. W., and Wardlaw,J. C. 1993. Contest competition among Maculinea rebeli butterfly larvae in ant nests. Ecol. Entomol. 18:73-76.
Thomas,J. A., Elmes,G. W., and Wardlaw,J. C. 1998. Polymorphic growth in larvae of the butterfly Maculinea rebeli,a social parasite of Myrmica ant colonies. Proc. R. Soc. London Ser. B-Biol. Sci. 265:1895-1901.
Thomas,J. A., Elmes,G. W., Wardlaw,J. C., and Woyciechowski,M. 1989. Host specificity among Maculinea butterflies in Myrmica ant nests. Oecologia 79:452-457.
Vander Meer,R. K., and Morel,L. 1998. Nestmate recognition in ants, pp. 79-103, in: R. K. Vander Meer, M. D. Breed, M. L. Winston, and K. E. Espelie) (eds.). Pheromone Communication in Social Insects. Westview Press, Boulder, Westriew Press, Boulder, Co.
Viana,A. M. M., Frezard,A., Malosse,C., Della Lucia,T. M. C., Errard,C.,and Lenoir,A. 2001. Colonial recognition of fungus in the fungus-growing ant Acromyrmex subterraneus (Hymenoptera: Formicidae). Chemoecology 11:29-36.
Wardlaw,J. C., Elmes,G. W., and Thomas,J. A. 1998. Techniques for studying Maculinea butterflies I: Rearing Maculinea caterpillars with Myrmica ants in the laboratory. J. Insect Conserv. 2:79-84.
Wardlaw,J. C., Thomas,J. A., and Elmes,G. W. 2000. Do Maculinea rebeli caterpillars provide vestigial mutualistic benefits to ants when living as social parasites inside Myrmica ant nests? Entomol. Exp. Appl. 95:97-103.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schönrogge, K., Wardlaw, J.C., Peters, A.J. et al. Changes in Chemical Signature and Host Specificity from Larval Retrieval to Full Social Integration in the Myrmecophilous Butterfly Maculinea rebeli . J Chem Ecol 30, 91–107 (2004). https://doi.org/10.1023/B:JOEC.0000013184.18176.a9
Issue Date:
DOI: https://doi.org/10.1023/B:JOEC.0000013184.18176.a9