Abstract
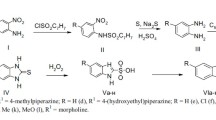
The “inactive metabolite approach” was used to design β-blockers. The acidic inactive metabolite of metoprolol [4-(2-hydroxy-3-isopropylamino) propoxyphenylacetic acid] was used as the lead compound. Its esters (alkyl and cycloalkyl) were found active in vivo while reverting quantitatively to the same inactive metabolite in plasma. The cyclohexyl ester showed the best activity, which was cardioselective, similar to the parent compound metoprolol. Although most esters had a plasma half-life of approximately 1 min, their activity (antagonism of isoproterenol induced increase in heart rate) following intravenous administration lasted 45–90 minutes, and the maximum β-blockade was observed at 45–60 minutes in both rats and dogs.
Similar content being viewed by others
References and Notes
Part V of this series: Bodor, N., Sloan, K. B. (1982) J. Pharm. Sci., 71, 514–520
After the present work was completed, a paper was published (Erhardt, P. W., Woo, C. M., Anderson, W. G., Gorczynski, R. J. [1982] J. Med Chem., 25, 1408–1412), describing some similar types of short acting β-blockers, among which was the methyl ester of the lead compound used in the present work.
Francis, R. J., East, P. B., McLaren, S. J., Larman, J. (1976) Biomed. Mass. Spectrometry, 3, 281–285.
Bodor, N., Proceedings of the 2nd IUPAC-IUPHAR Symposium on Strategy in Drug Research, Noordwijkerhout, J. A. Keverling Buisman (ed.), Elsevier Scientific Publishing Company, Amsterdam, 1982.
Bodor, N. Belg. Pat. 839, 563, 03 Nov. 1981. Chem Abstr. 97: 6651 n (1982) and Bodor, N., Little, R. J., unpublished results.
Borg, K. O., Carlsson, E., Hoffman, K. J., Jönsson, T. E., Thorin, H., Wallin, B. (1975) Acta Pharmacol. Toxicol., 36 (Suppl. V), 125–135.
Åblad, B., Borg, K. O., Johansson, G., Regårdh, C. G., Sölvell, L. (1975) Life Sciences, 14, 693–704.
Hoffman, K. J., Regårdh, C. G., Avrell, M., Ervik, M., Jordö, L. (1980) Clin. Pharmacokin., 5, 181–191.
Regårdh, C. G., Ek. L. and Hoffman, K. J. (1979) J. Pharmacokin. Biopharm., 7, 471–479.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bodor, N., Oshiro, Y., Loftsson, T. et al. Soft Drugs VI. The Application of the Inactive Metabolite Approach for Design of Soft β-Blockers1?2. Pharm Res 1, 120–125 (1984). https://doi.org/10.1023/A:1016376003515
Issue Date:
DOI: https://doi.org/10.1023/A:1016376003515