Abstract
Purpose. To develop a predictive algorithm of nonelectrolyte transport through skin based upon a partitioning-diffusion model.
Methods. Drug permeability is described by a partitioning-diffusion equation. Through free-energy relationships, partitioning is related to the drug's molecular volume (MV), and hydrogen bond donor (Hd) and acceptor (Ha) activity. Diffusion is related to the drug's MV using a theory of diffusion through lipid lamellae based on free-volume fluctuations within the lipid domain. These two explicit descriptions are combined to give an equation describing permeability in terms of the permeant's physical properties. The aqueous permeability coefficients of 37 nonelectrolytes through human epidermis were evaluated as a function of these physical properties using a multiple regression analysis.
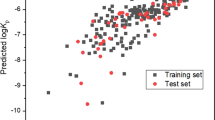
Results. The results of the regression analysis show that 94% of the variability in the data can be explained by a model which includes only the permeant's MV, Hd and Ha. These results further provide an algorithm to predict skin permeability based upon the values of these parameters. In addition, the relative contribution of various chemical functional groups (e.g., -COOH) is derived, and can be used to predict skin transport from drug structure alone.
Conclusions. A biophysically relevant model of drug transport through human skin is derived based solely on the physical properties of the drug. The model provides an algorithm to predict permeability from the drug's structure and/or physical properties. Moreover, the model is applicable to a number of lipid barrier membranes, suggesting a common transport mechanism in all.
Similar content being viewed by others
REFERENCES
G.B. Kasting, R.L. Smith, E.R. Cooper. Effect of lipid solubility and molecular size on percutaneous absorption. In Skin Pharmacokinetics, B. Shroot and H. Schaefer (eds.), Karger, Basel, pp. 138–153 (1987).
B.D. Anderson, W.I. Higuchi, P.V. Raykar. Heterogeneity effects on permeability-partition coefficient relationships in human stratum corneum. Pharm. Res. 5: 566–573, 1988.
B.D. Anderson, W.I. Higuchi, P.V. Raykar. Solute structure-permeability relationships in human stratum corneum. J. Invest. Dermatol. 93: 280–286, 1989.
G.L. Flynn. Physicochemical determinants of skin absorption. In Principles of Route-to-Route Extrapolation for Risk Assessment., TR Gerrity and CJ Henry (eds.), Elsevier, New York, pp. 93–127, 1990.
N. El Tayar, R-Y. Tsai, B. Testa, P-A. Carrupt, C. Hansch, A. Leo. Percutaneous penetration of drugs: A quantitative structure-permeability relationship study. J. Pharm. Sci., 80: 744–749, 1991.
R.O. Potts, R.H. Guy. Predicting skin permeability, Pharm. Res., 9: 663–669, 1992.
W.J. Pugh, J. Hadgraft. Ab initio prediction of human skin permeability coefficients. Inter. J. Pharm. 103: 163–178, 1994.
N. El Tayar, R-Y. Tsai, B. Testa, P-A. Carrupt, A. Leo. Partitioning of solutes in different solvent systems: The contribution of hydrogen-bonding capacity and polarity. J. Pharm. Sci., 80: 590–598, 1991.
M.J. Kamlet, J-L.M. Abboud, M.H. Abraham, R.W. Taft. Linear solvation energy relationship: 23: A comprehensive collection of the solvatochromatic parameters p, a and b and some methods for simplifying the generalized solvatochromatic equation. J. Org. Chem., 48: 2877–2887, 1983.
M.J. Kamlet, R.M. Doherty, J-L.M. Abboud, M.H. Abraham, R.W. Taft. Linear solvation energy relationships: 36. Molecular properties governing solubilities of organic nonelectrolytes in water. J. Pharm. Sci., 75: 338–349, 1896.
M.H. Abraham, P.L. Grellier, D.V. Prior, P.P. Duce, J.J. Morris, P.J. Taylor. Hydrogen bonding. Part 7. A scale of solute hydrogen-bond acidity based on log K values for complexation in tetrachloromethane. J. Chem. Soc. Perkin Trans., II: 699–711, 1989.
M.H. Abraham, P.L. Grellier, D.V. Prior, J.J. Morris, P.J. Taylor. Hydrogen bonding. Part 10. A scale of solute hydrogen bond basicity using log K values for complexation in teetrachloromethane. J. Chem. Soc. Perkin Trans., II: 521–529, 1990.
M.H. Abraham. Application of solvation equations to chemical and biochemical processes. Pure & Appl Chem., 65: 2503–2512, 1993.
M.H. Abraham, H.S. Chadha, R.C. Mitchell. Hydrogen bonding part 33; The factors that influence the distribution of solutes between blood and brain. J. Pharm. Sci., 83: 1257–1268, 1994.
R. Collander,. The partitioning of organic compounds between higher alcohols and water. Acta Chem. Scand., 5: 774–780, 1951.
M.H. Cohen, D. Turnbull. Molecular transport in liquids and glasses. J. Chem. Phys., 31: 1164–1168, 1959.
H. Trauble. The movement of molecules across lipid membranes: A molecular theory. J. Memb. Biol., 4: 193–208, 1971.
W.R. Lieb, W.D. Stein. Non-Stokesian nature of transverse diffusion within red cell membranes. J. Memb. Biol., 92: 111–119, 1986.
W.R. Lieb, W.D. Stein. In “Transport and Diffusion across Cell Membranes”, W.D. Stein, Academic Press, New York, 1986.
K.A. Dill, J. Naghizadeh, J.A. Marqusee. Chain molecules at high densities at interfaces. Ann. Rev. Phys. Chem., 39: 425–461, 1988.
T-X. Xiang, C. Xueling, B.D. Anderson. Transport methods for probing the barrier domain of lipid bilayers. Biophys. J., 63: 78–88, 1992.
T-X. Xiang, B.D. Anderson. Molecular distributions in interphases: Statistical mechanical theory combined with molecular dynamics simulation of model lipid bilayers. Biophys. J., 66: 561–573, 1994.
A. Bondi. van der Waals volumes and radii. J. Phys. Chem., 68: 441–451, 1964.
C. Ackermann, G.L. Flynn, W.M. Smith. Ether-water partitioning and permeability through hairless mouse skin in vitro. II. Hydrocortisone 21-n-alkyl ester, alkanol and hydrophilic compounds. Int. J. Pharm., 36: 67–71, 1987.
C.R. Sanders, J.P. Schwonek. An approximate model and empirical energy function for solute interactions with a waterphophatidykcholine interface. Biophys. J., 65: 1207–1218, 1993.
R.J. Scheuplein, I.H. Blank. Permeability of the skin. Physiol. Rev., 51: 702–747, 1971.
R.L. Cleek, A.L. Bunge. A new method for estimating ermal absorption from chemical exposure. I. General approach. Pharm. Res., 10: 497–506, 1993.
G.B. Kasting, P.J. Robinson. Can we assign an upper limit to skin permeability? Pharm. Res., 10: 930–931, 1993.
M.H. Abraham, H.S. Chadha, R.C. Mitchell. The factors that influence skin penetration of solutes. J. Pharm. Pharmacol., 47: 8–16, 1995.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Potts, R.O., Guy, R.H. A Predictive Algorithm for Skin Permeability: The Effects of Molecular Size and Hydrogen Bond Activity. Pharm Res 12, 1628–1633 (1995). https://doi.org/10.1023/A:1016236932339
Issue Date:
DOI: https://doi.org/10.1023/A:1016236932339