INTRODUCTION
The potential consequences of the European Union ban of antimicrobial growth promoters (AGPs) [1] on both animal and human health have recently been reviewed [Reference Casewell2, Reference Phillips3]. One negative consequence of the ban may be increased disease in pigs, leading to an increased use of therapeutic antibiotics of importance in human medicine [Reference Casewell2].
Since the 1970s, antimicrobials have been used as growth promoters for pigs in Denmark. However, in early 1998, the Danish Bacon and Meat Council, representing over 95% of Danish pig producers, agreed to phase out the use of AGPs because of the public concern. The concern was that the use of antimicrobials for growth promotion could lead to selection of resistant bacteria that were pathogenic to humans. A detailed description of the chronology of the phasing out of AGPs in Danish pig production is available in a WHO report on the Danish experiences of discontinued use of AGPs (duAGPs) in animal production [4].
Prior to the withdrawal of AGPs, nearly all pigs in Denmark reared for consumption were continuously exposed to antimicrobials until a few weeks before slaughter. From the time of weaning until an age of 4 months, pigs were given one of the following AGPs in feed: tylosin, 10–40 ppm; olaquinodox, 15–50 ppm; carbadox, 20–50 ppm; or avilamycin, 20–40 ppm. From 4 months of age until 4 weeks prior to slaughter (at approximately 6 months of age), the most frequently given AGP was tylosin, but at a lower concentration (5–20 ppm).
Since 1 January 2000, all use of AGPs has been discontinued in Danish pig production. The withdrawal of AGPs from pig production was performed in two steps. At the end of 1998, the use of AGPs was discontinued for pigs >35 kg, and in 1999, the pig producers also agreed on terminating the use of AGPs for pigs <35 kg by the end of that year. Therefore, in this study we define pigs after weaning until a weight of 35 kg (age of ~14 weeks) as weaners and pigs >35 kg are defined as finishers.
A nationwide withdrawal of AGPs from pig production has previously only been performed in Sweden in 1986 and in Switzerland in 1999. At national level the duAGPs in Sweden did not create obvious clinical problems for finishers, whereas significant problems emerged temporarily among weaners (increased mortality and disease incidence) [Reference Wierup5]. In the Swiss study no increase in the amount of prescribed antibiotics after duAGPs could be detected in an evaluation of more than 6000 prescriptions made over 6 years (1996–2001) [Reference Arnold6]. In a study of 29 pig farms in Finland, only four farms had an increased use of antibiotics after duAGPs [Reference Laine7].
The overall goal of this study was to evaluate the effect of duAGPs on the frequency of disease in Danish pig farms. This was done by comparing the frequency of antibiotic treatment before and after withdrawal of AGPs in 68 conveniently sampled farrow-to-finish pig farms. However, it must be emphasized that recording treatment is an indirect method of recording occurrence of disease. The effect of duAGPs was measured on (1) proportion of days per farm where treatment was performed, and (2) proportion of pigs treated per day per farm at days where treatment was performed.
The use of AGPs to finishers had already ceased before the start of the study. Therefore, it must be underlined that in this study we assess the effect of duAGPs to weaners (that is no AGPs were used from weaning to slaughter) on the risk of therapeutic antibiotic treatment of pigs from weaning to slaughter. It was not possible to estimate the effect only in weaners, because the study farms did not have the same movement patterns between weaning and finishing units and therefore the age of pigs within these units was not directly comparable between farms. If nothing else is stated, duAGPs refers to the discontinued use of AGPs for pigs <35 kg.
MATERIAL AND METHODS
Study design and study population
The effect of duAGPs in weaners on the risk of antibiotic treatment of pigs from weaning to slaughter was estimated in a non-randomized case-crossover study. In the study farms, treatments were recorded as treatments given either in the weaning unit or finishing unit. However, as mentioned in the Introduction, because the farms did not have the same movement patterns between weaning and finishing units the age of pigs within these units was not directly comparable between farms. Therefore, the estimated effect of duAGPs in weaners is an overall effect on pigs from weaning to slaughter. Each farm passed through both the non-exposed time period (use of AGPs to weaners) and the exposed time period (no use of AGPs to weaners), and therefore each farm served as its own control. No estimates of the effect of duAGPs existed a priori for calculation of sample size. However, as we expected that the effect of duAGPs would vary between farms, 150 farms were chosen as a presumably sufficient number for estimation of an average effect and a between-farm variation in the effect.
The study population was obtained using a two-stage sampling scheme [(1) veterinarian; (2) farms within veterinarian]. Contact with 16 veterinarians, specializing in pig diseases and distributed throughout Denmark, was established. Each veterinarian was asked to nominate farm operations to which their practice routinely provided medical care. The veterinarian was required, as first priority, to nominate farrow-to-finish operations, and as a second priority to nominate operations where one sow-farm traded pigs to one single-finish-farm, that only received pigs from the sow-farm, and both farms should be willing to participate in the study. For simplicity, an operation will be referred to as a ‘farm’ throughout this paper, even though the finishers were located on a finish-farm, apart from the sows and weaners. All farms included in the study represented semi-closed pig populations, as only breeding stock entered the farms.
Since each farm served as its own control, a reliable measure of frequency of treatment both before and after duAGPs in each farm was necessary. Therefore, farms where treatments had been recorded for <8 weeks, either before or after duAGPs, were excluded from the analysis. The 8 weeks' cut-off was subjectively selected. Of the 150 farms selected for the study, 134 were enrolled and 68 had complete and reliable data, so they could be included in the analyses (Fig. 1). Fourteen different veterinarians managed the farms included in the analysis.

Fig. 1. Diagram illustrating the reduction of number of farms included in the data analysis estimating the effect of discontinued use of antimicrobial growth promoters (duAGPs) on the risk of antibiotic treatment in Danish pig production.
Data collection
Date of duAGPs
Initially, for each farm a questionnaire was applied to obtain the date of duAGPs to weaners. Thereafter, the date of discontinued use was confirmed by the feed mill that delivered the feedstuff or the premix containing AGPs (to add to the homemade feed).
Data on management factors
The same questionnaire was applied to collect farm-specific data on management factors that may interact with the effect of duAGPs on risk of treatment. The questionnaire was designed to collect data about each production unit (farrowing unit, weaning unit, second weaning unit if present, growing unit if present, finishing unit). The pig producer and the veterinarian completed the questionnaire together. The questionnaire encompassed questions about the production facilities and the management of pigs (Tables 1 and 2). In addition, the veterinarians were asked to report, at monthly intervals, important changes in the management of the farms (e.g. expansion, rebuilding, interventions against diseases).
Table 1. Description and distribution of 20 categorical management factors. Data collected from 68 Danish farrow-to-finish pig farms (1998–2002)
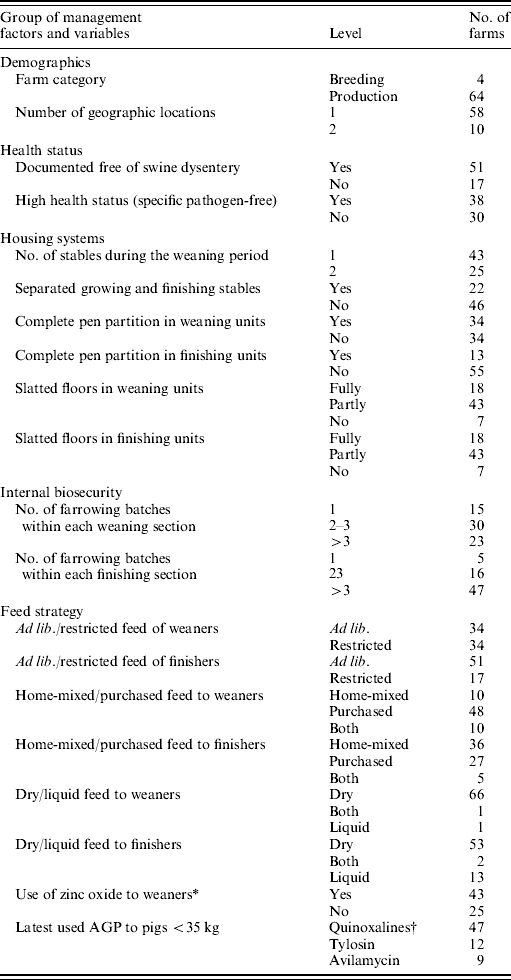
* 250 ppm zinc in pig diets.
† Carbadox, olaquinodox.
Table 2. Description and distribution of five continuous management factors. Data collected from 68 Danish farrow-to-finish pig farms (1998–2002)
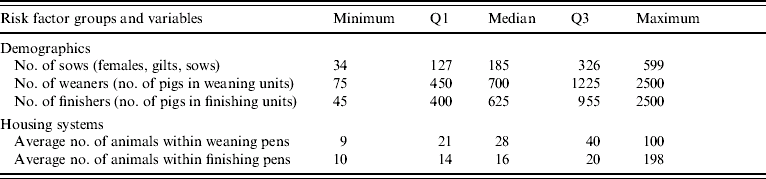
Q1, First quartile; Q3, third quartile.
Data about antibiotic treatment and disease
The pig producers (owner and stable staffs) recorded data about antibiotic treatment on a specially designed form (available in Danish on request). In the study farms, the dates for the first recorded antibiotic treatment ranged from 10 December 1998 to 3 July 1999 and the dates for the last recorded antibiotic treatment ranged from 15 December 1999 to 1 February 2002. For each event of antibiotic treatment, date, clinical signs treated, production unit and number of animals treated were recorded. We defined five diseases [diarrhoea, arthritis, pneumonia, unthriving and miscellaneous disorders (e.g. tail biting, meningitis, otitis media and dermatitis)] by the clinical signs observed by the pig producers.
Statistical analysis
The aim of the statistical analysis was to estimate the effect of duAGPs on the risk of antibiotic treatment of pigs from weaning to slaughter specifically: (1) were pigs treated on more days after duAGPs than before? (2) On days when pigs were treated, were more pigs treated than before? By pairing each farm with itself in the case-crossover design management factors were eliminated as confounders [Reference Greenland8]. Therefore, the effects of management factors were considered as interaction factors only. Data included in the statistical analysis to define treatment level within the farms before and after duAGPs were restricted to the data recorded within the last year before discontinuation and first year after discontinuation, respectively. Therefore, the estimated effect of duAGPs is the average effect during the first year after duAGPs. According to the aim of the analysis, the effect of duAGPs was estimated in two separate analyses using two different response variables; ‘effect on proportion of days per farm where treatment was performed’ (analysis no. 1), and ‘effect on proportion of pigs treated per day per farm at days where treatment was performed’ (analysis no. 2), respectively.
In all analyses, the effect of duAGPs was evaluated for diarrhoea, arthritis, pneumonia, unthriving and miscellaneous disorders, separately. The effect was measured as the odds ratio (OR), defined as the ratio between the odds for treatment after discontinuation and the odds for treatment before discontinuation.
We expected a high within-farm correlation of the management factors, and attempts were made to identify latent farm characteristics in the data using factor analysis. The collected information was organized and transformed (optimal scaling) in a way that made factor analysis possible. In the factor analysis, the overall value of Keiser's measure of sampling adequacy (measuring how much smaller the partial correlations are in the factor solution compared to the original correlations) was 0·46, indicating that the collected data was not appropriate for factor analysis (<0·5 is unacceptable [9]). Thus, the original variables of management factors were used in the statistical analysis.
Effect on proportion of days per farm where treatment was performed (analysis no. 1)
The epidemiological unit of interest in analysis no. 1 was the farm. The dependent variable – proportion of days per farm where treatment was performed (PDT) – was a binomial proportion, with the number of days where antibiotic treatment was performed in the numerator and the number of days in study in the denominator:
The number of days in study was defined by the number of days between the first and last recorded treatment at each farm. Two proportions per farm were created to reflect the study periods ‘before’ and ‘after’ duAGPs.
Effect on proportion of pigs treated per day per farm at days where treatment was performed (analysis no. 2)
The epidemiological unit of interest in analysis no. 2 was day were treatment was performed within each farm. The dependent variable – proportion of pigs treated per day per farm at days where treatment was performed (PPT) – was a binomial proportion, with the number of pigs treated with antibiotics per day per farm at days where treatment was performed in the numerator and the sum of the number of weaners and finishers (given in the questionnaires) at the farm in the denominator.
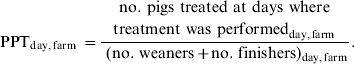
A proportion for each day where antibiotic treatment was performed per farm was created.
Modelling
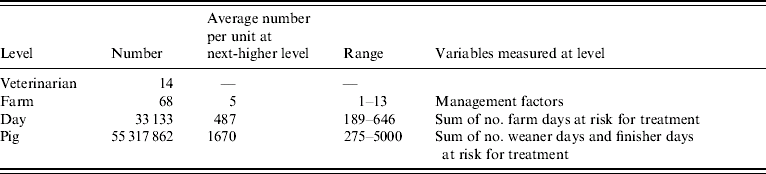
The data had a hierarchical structure (Table 3). Both PDT and PPT were clustered in two dimensions – within time and within space (days and animals, respectively, within farm and farm within veterinarian). Procedures for estimating parameters in a multilevel model including repeated measurements with binomially distributed dependent variable are not well established. Therefore, first multilevel logistic regression models, with random intercepts, were set up in order to estimate the effect of duAGPs on the PDT [three levels: veterinarian, farm, day (Table 3)] and on PPT [four levels: veterinarian, farm, day, pig (Table 3)]. The initially specified logistic models included no covariance between the measures at lowest level (days and pigs, respectively), assuming independence between measures. In the models, the effects of duAGPs on PDT and PPT were estimated as a fixed effect and a between-farm Gaussian-distributed random effect (random slope).
Table 3. Four-level hierarchical structure of data used to estimate the effect of duAGPs on the risk of therapeutic antibiotic treatment in Danish farrow-to-finish farms
In general, the following procedure was used to fit the models. First, the unconditional association between duAGPs and the dependent variable was estimated using logistic regression where no extra-binomial variation was permitted. By unconditional association between duAGPs and the dependent variables we understand the association (fixed and random effects) between the duAGPs and the dependent variable when no other explanatory variables were included in the model.
Second, in the unconditional models presence of extra-binomial variation was examined by estimating an additional dispersion parameter monitoring the variability of the binary outcome relative to a binomial distribution in the models. Concerning PDT, for all diseases the estimate of day-level variance remained very close to 1 (>0·9), indicating that the assumption about binomial distribution was fulfilled. However, in models for PPT, only for diarrhoea did the estimated extra-binomial variation remain close to 1. For the other diseases, the data were under-dispersed (0·0–0·6). A potential cause of under-dispersion in this study is the presence of dependency between adjacent measures of PPT within farms (autocorrelation). Therefore, the assumption of independent residuals was replaced by the assumption of first-order autocorrelation, which represents a type of dependence between adjacent observations, which dies out between observations that are further apart. This was done in a linear multilevel regression, with PPT treated as a continuous variable, taking into account both the within-veterinarian and within-farm clustering and within-time clustering. The within-veterinarian and within-farm clustering were adjusted by including an intercept with random effects at veterinarian level and farm level (similar to the case in the logistic model), and the clustering in time was adjusted for by using a spatial-power covariance structure. Since the distribution of PPT was skewed, PPT was transformed using logarithmic transformation.
Third, presence of interaction between the effect of duAGPs and management factors was evaluated by adding each management factor separately to the unconditional models as a fixed main effect and a fixed first-order interaction term with the fixed effect of duAGPs. The significance of the main effect and the interaction was evaluated by Wald's test (P<0·05). Potential interaction factors with more than two categories were evaluated using dummy variables and Bonferroni correction for multiple testing (P<0·05/k, where k is the total number of dummy variables).
To obtain a final estimate of the effect of duAGPs (average effect during the first year after duAGPs), management factors identified as being associated with the dependent variable were added to the unconditional model simultaneously. Non-significant (Wald's test, P>0·05) interaction terms and thus main effects were removed sequentially. Finally, significance of the random effect of duAGPs was tested using likelihood ratio tests. Throughout the modelling we retained the random effects of the intercept at all levels in the in the models, even if the estimates were very close to zero.
In addition, the effect of the interaction between time elapsed after discontinuation and effect of duAGPs (time-dependency of the effect of duAGPs) was estimated. The time-period after discontinuation (1 year) was divided into monthly (30 days) intervals (12 dummy variables). The interaction term (duAGPs×time-period after discontinuation) added to the final model estimated the average effect during the first year after duAGPs.
Estimation methods
Second-order penalized quasi-likelihood (PQL-2) estimates were used to build the logistic models in MLwiN [Reference Rasbash10]. Revised estimates for logistic multilevel models of PDT were obtained using Markov-chain Monte Carlo (MCMC) and maximum-likelihood (ML) estimation. The MCMC estimation was done using Metropolis–Hastings sampling with diffuse priors implemented in MLwiN, with a burn-in period of 5000 iterations and a run of 50 000 iterations. The ML estimates were derived using Gaussian adaptive quadrature integration using the Stata program gllamm [Reference Rabe-Hesketh, Skrondal and Pickles11]. The linear multilevel models of PPT were fitted to the data using the method of restricted maximum likelihood (REML) implemented in the mixed procedure in SAS [9].
In ML-estimated logistic multilevel models the adequacy of the assumptions of Gaussian-distributed random effects were evaluated by the Normality probability plots of empirical Bayes estimates of the random effects (plots not shown). In the ML-estimated linear multilevel models the adequacy of the assumptions of Gaussian-distributed random effects and residuals were evaluated by the Normality probability plots of empirical best-linear unbiased predictors of the random effects (plots not shown).
For diseases on which the withdrawal of AGPs had an effect on PDT, the within-farm correlation of the frequency of antibiotic treatment before duAGPs and the effect of duAGPs was calculated as:
where covariance is the covariance between intercept and duAGPs, varianceintercept is the between-farm variance of the intercept and varianceduAGPs is the between-farm variance of the effect of duAGPs.
For diseases on which the withdrawal of AGPs had an effect on both PDT and PPT, the association between the farm-specific effects of duAGPs on PDT and PPT was evaluated by the Spearman rank-order correlation. The farm-specific effect (OR) on PDT was predicted using empirical Bayes estimation, which for each farm combined the data obtained in that farm and the population information estimated in the statistical model [Reference Snijders and Bosker12]. Farm-specific values of PPT before and after discontinuation were predicted from the final linear model, using the estimate of the fixed effect and empirical best-linear unbiased predictors of the random effects. The farm-specific effect (OR) on PPT was estimated by the ratio between PPT after and before duAGPs.
RESULTS
Descriptive statistics
Management factors
The distributions of the 25 management factors on the 68 farms included in the analyses are presented in Tables 1 and 2.
Study variable
In the study farms, the date for duAGPs for weaners ranged from 1 April 1999 to 25 February 2000 (1st quartile=1 August 1999; median=31 August 1999; 3rd quartile=14 September 1999). The maximum number of days a farm was included in the study before duAGPs was 12 months (1st quartile, 6·9 months; median, 5·5 months; 3rd quartile, 4·2 months, minimum 2·2 months). The maximum the 1st quartile and the median number of days a farm was included in the study after duAGPs was 12 months; the 3rd quartile was 10·1 months maximum and 3·5 months minimum.
Response variable
In Figure 2 the mean of farm-specific daily proportion of pigs treated (no. treated animalsday,farm/no. weaners and finishersfarm) for diarrhoea, arthritis, pneumonia, unthriving and miscellaneous disorders, respectively is plotted against time which is centred around the date of duAGPs. The incidence density of antibiotic treatment of weaners and finishers, respectively, before and after duAGPs stratified by disease is presented in Table 4. The shape of the graphs (Fig. 2) and the calculated incidence densities (Table 4) indicate that the most obvious effect of duAGPs was on the treatments for diarrhoea – both among weaners and finishers.
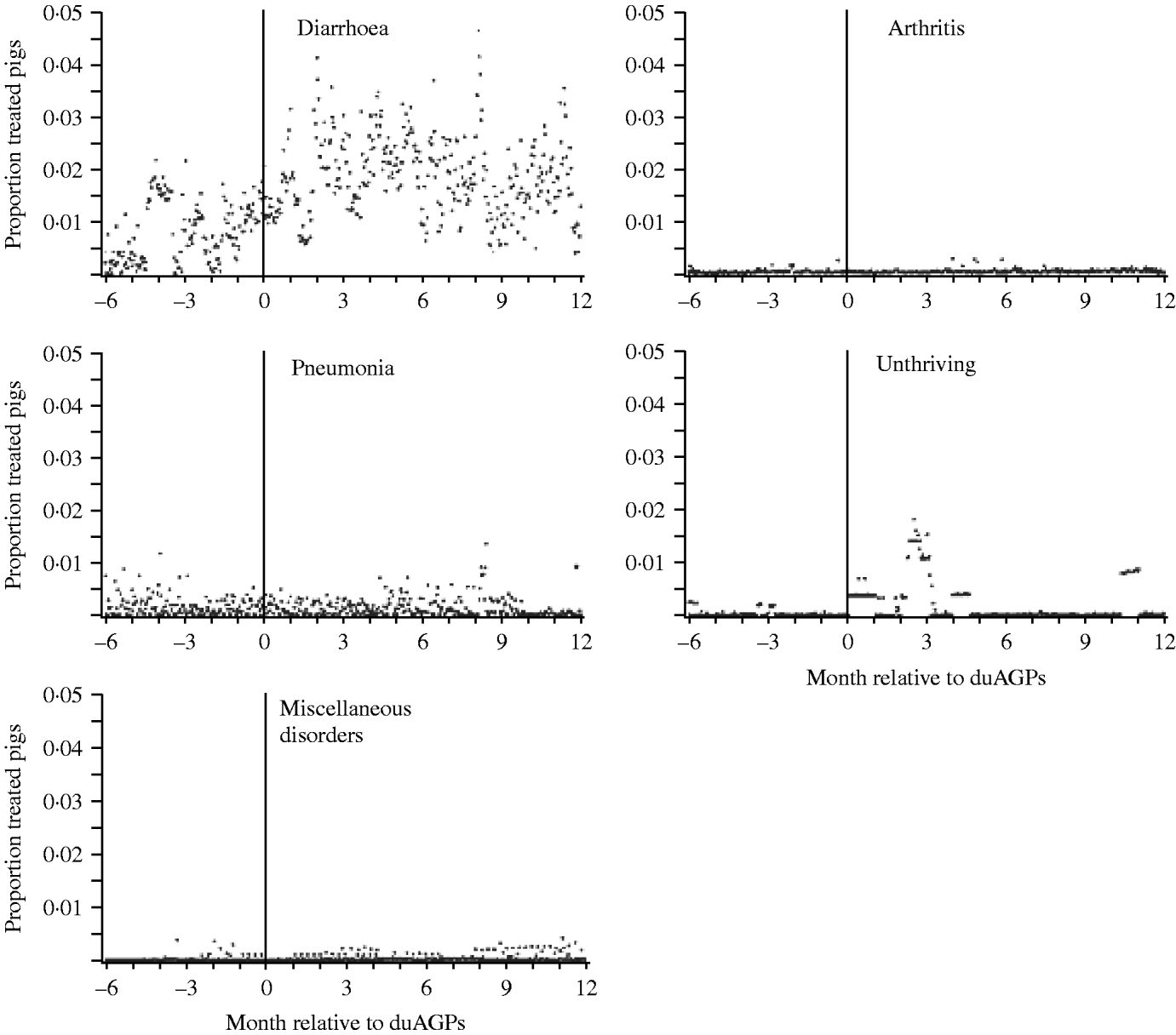
Fig. 2. Mean of farm-specific daily proportion of pigs (weaners and finishers) treated for diarrhoea, arthritis, pneumonia, unthriving and miscellaneous disorders, respectively, plotted against time before and after discontinued use of antimicrobial growth promoters (duAGPs). Based on data collected before and after duAGPs in 68 Danish farrow-to-finish pig farms (1998–2002). Due to the small number of farms contributing data in the period several months before duAGPs, the horizontal axis was truncated at – 6 months (the median of days in study per farm before duAGPs).
Table 4. Frequency (n) and incidence density (ID) of antibiotic treatments of weaners and finishers before and after the duAGPsFootnote *, respectively. Stratified by disease. Data collected before and after duAGPs in 68 Danish farrow-to-finish pig farms (1998–2002)
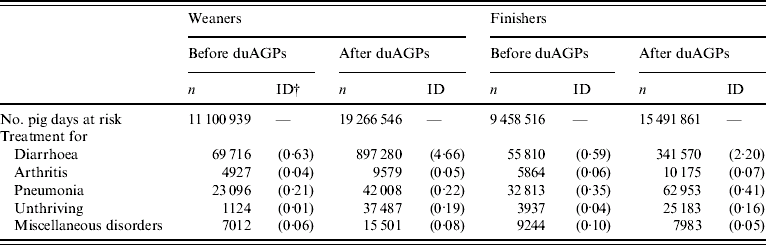
* Discontinued use of antimicrobial growth promoters.
† Incidence density (no. antibiotic treatments per 100 pig days).
The distribution of PDT diarrhoea after duAGPs indicated a large variation between farms (minimum=0%; 1st quartile=5%, median=17%; 3rd quartile=30%, maximum=93%), e.g. some farms never or rarely treated for diarrhoea, whereas some farms treated for diarrhoea more than one third of the days. The distribution of PPT diarrhoea after duAGPs (minimum=0·1%; 1st quartile=0·5%, median=2·6%; 3rd quartile=13%, maximum=100%) indicated that sometimes diarrhoea was treated using individual medication (very low percentage of pigs treated) whereas in other cases diarrhoea was treated using batch medication (moderate and high percentage of pigs treated).
Results of the statistical analyses
For MCMC estimation in analysis no. 1, the sampling traces for the parameters looked healthy in all models, and convergence of the Markov chain to the posterior distribution was observed. In the ML estimation, the criteria for convergence were achieved, and the correlations between parameter estimates were low. The assumption about Gaussian-distributed random effects was satisfied in all models. The revised parameter estimates obtained using MCMC estimation (posterior mean and 95% credibility intervals) and ML estimation (point estimate and 95% Wald-type confidence intervals) where very similar in all models.
In analysis no. 2 the linear multilevel model allowing for autocorrelation between adjacent measures gave a better fit when compared to the model assuming independency between adjacent measures. The assumption about Gaussian-distributed random effects was satisfied in all models.
If nothing else is stated, the estimates obtained using ML estimation (PDT) and REML estimation (PPT) are presented.
For none of the response variables did the effect of duAGPs show significant interaction with any of the management factors. Therefore, the unconditional estimated effect of duAGPs on PDT (logistic regression) and on PPT (linear regression), respectively, were the final estimates (Tables 5 and 6). A significant effect was identified on diarrhoea, both for PDT (OR 2·5, 95% CI 1·7–3·8) and PPT, whereas the risk of antibiotic treatment for other diseases was unaffected by withdrawal. The MCMC estimated OR of the effect of duAGPs on PPT diarrhoea obtained in the four-level logistic regression was 1·6 (95% credibility interval of OR 1·4–2·2). Concerning PDT diarrhoea there was a small negative within-farm correlation [correlation coefficient=−0·35 (Table 5)] between the frequency of antibiotic treatment for diarrhoea before duAGPs and the effect of duAGPs.
Table 5. Maximum-likelihood estimates of three-level logistic models estimating the effect of duAGPsFootnote † on the proportion of days per farm where treatment was performed (PDT) (analysis no. 1). Based on data collected before and after duAGPs in 68 Danish farrow-to-finish pig farms (1998–2002)
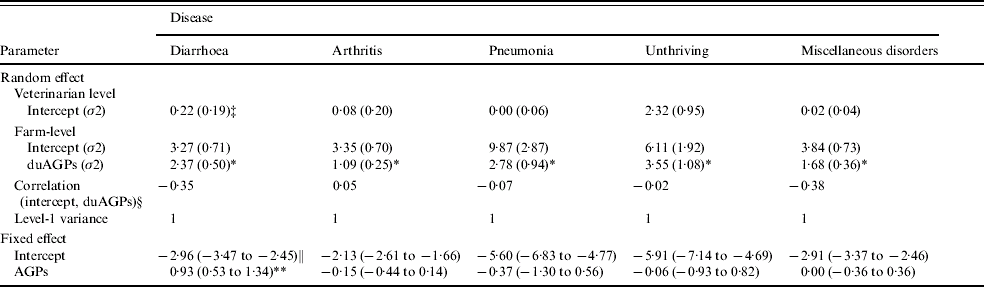
† The average effect during the first year after discontinued use of antimicrobial growth promoters.
‡ Standard errors of random effect estimate in parenthesis.
§ Correlation between the effect of duAGPs and proportion of days where treatment was performed before duAGPs.
∥ 95% Wald-type confidence interval of fixed effect estimate in parentheses.
* Statistical significant random effect of duAGPs (likelihood ratio test, P<0·05).
** Statistical significant fixed effect of duAGPs (Wald's test, P<0·05).
Table 6. Restricted maximum-likelihood estimates of four-level linear models estimating the effect of duAGPsFootnote † on the proportion of pigs treated per day per farm where treatment was performed (log PPT) (analysis no. 2). Based on data collected before and after duAGPs in 68 Danish farrow-to-finish pig farms (1998–2002)
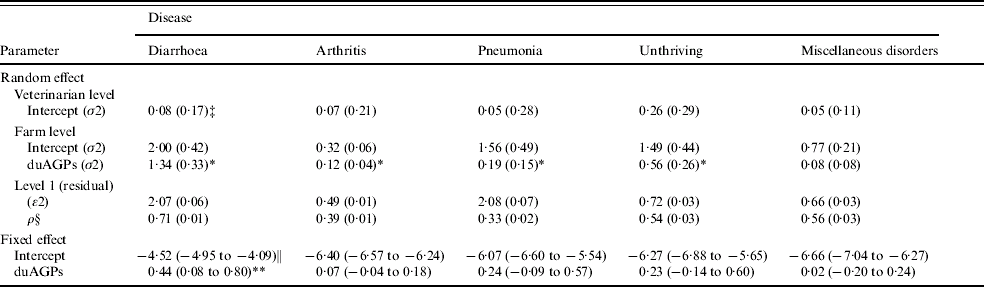
† The average effect during the first year after discontinued use of antimicrobial growth promoters.
‡ Standard errors of random effect estimate in parentheses.
§ Within farm correlation between PPT in two adjacent days.
∥ 95% Wald-type confidence interval of fixed effect estimate in parentheses.
* Statistical significant random effect of duAGPs (likelihood ratio test, P<0·05).
** Statistical significant fixed effect of duAGPs (Wald's test, P<0·05).
In all models, except the PDT model of miscellaneous disorders, the farm random effect of duAGPs (the random slope) was found statistically significant (Tables 5 and 6), irrespective of the significance of the fixed effect of duAGPs.
According to the predicted farm-specific effect of duAGPs, ~10% of the study farms (7/68) experienced neither an increase in PDT diarrhoea nor PPT diarrhoea after discontinued use, whereas ~50% (36/68) of the farms experienced an increase in both PDT diarrhoea and PPT diarrhoea. The predicted farm-specific effect on PDT diarrhoea was plotted against the predicted farm-specific effect on PPT diarrhoea (Fig. 3). There was no significant correlation within farms between the effect of duAGPs on PDT and PPT regarding diarrhoea (correlation coefficient=0·14, P=0·27).
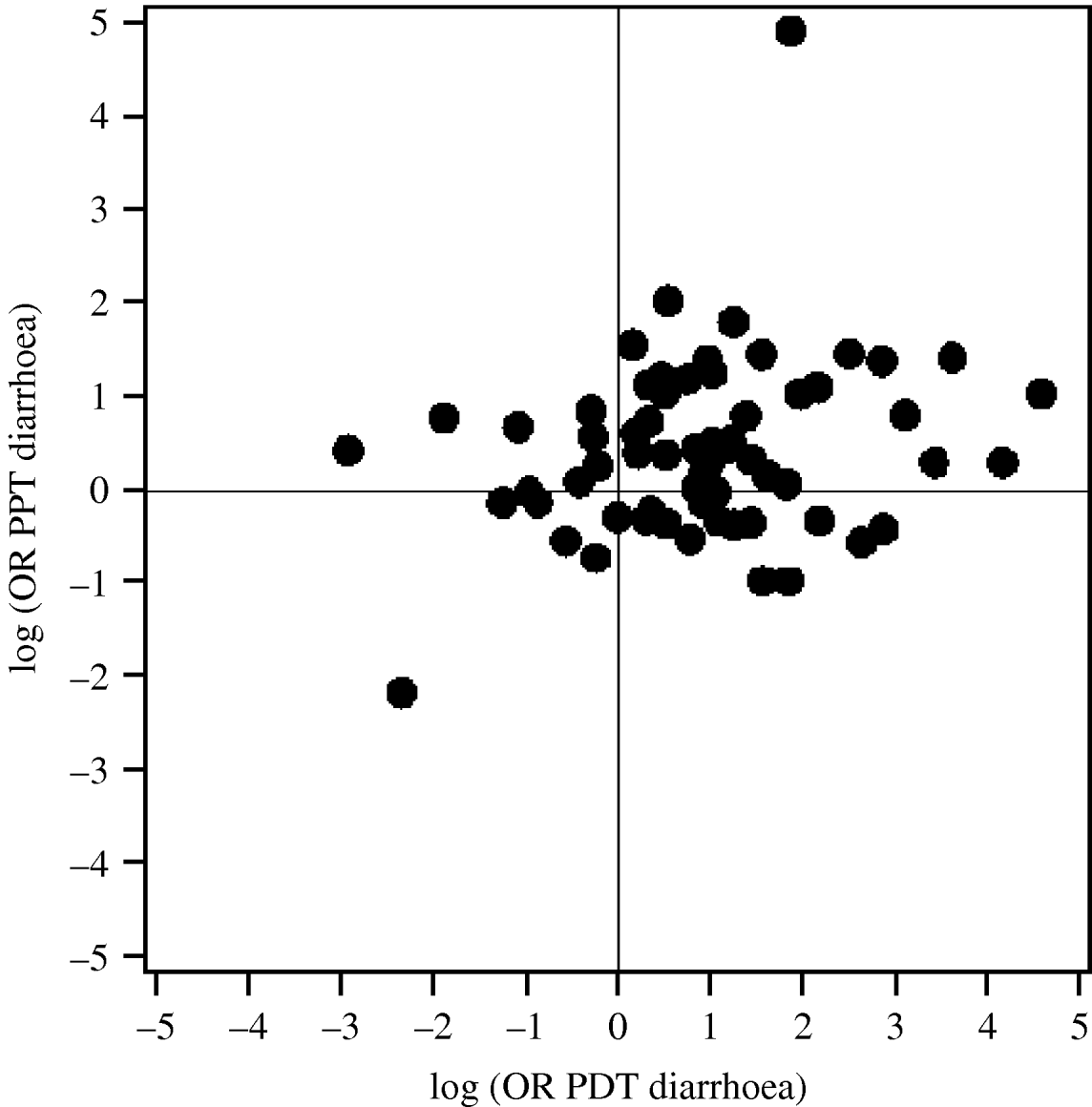
Fig. 3. The predicted farm-specific effect (log OR) of discontinued use of AGPs on PDT diarrhoea plotted against the predicted farm-specific effect (log OR) of discontinued use of AGPs on PPT diarrhoea in 65 Danish farrow-to-finish pig farms.
The estimated time-dependent effect of duAGPs on PDT diarrhoea indicated an effect of time elapsed after discontinuation. Compared to the period before duAGPs, increase in PDT diarrhoea was not significant within the first 3 months after discontinued use [1st month: OR 1·3 (99·5% CI 0·7–2·4); 2nd month: OR 1·6 (0·8–3·0); 3rd month: OR 1·9 (0·9–3·6)], whereas from the 4th month, the increase in PDT diarrhoea was statistically significant compared to the period before discontinuation [4th month: OR 2·4 (99·5% CI 1·3–4·5); 5th month: OR 2·9 (1·6–5·6); 6th month: OR 3·4 (1·8–6·5); 7th month: OR 3·2 (1·7–6·1); 8th month: OR 3·5 (1·8–6·5); 9th month: OR 2·8 (1·5–5·4); 10th month: OR 2·6 (1·4–5·1); 11th month: OR 3·4 (1·8–6·4); 12th month: OR 3·8 (2·0–7·2)].
DISCUSSION
To our knowledge, this is the first time the frequency of therapeutic antibiotic treatment of pigs before and after duAGPs has been recorded and analysed in an attempt to estimate the effect of duAGPs on the risk of treatment. The aim of this study was not to estimate the effect of other management procedures on the risk of antibiotic treatment.
Study design
By pairing each farm to itself using a case-crossover design, the risk that the estimated effect of duAGPs was confounded by constant farm-level management factors was eliminated [Reference Greenland8]. None of the farms reported that they directly implemented disease-preventing interventions as a reaction to duAGPs. However, it is likely that interventions were implemented in response to emerging disease problems, which may invalidate the assumption of constant exposure to farm-level management factors over time. Therefore, data of treatment after duAGPs included in the statistical analysis were restricted to the data recorded within the first year after discontinuation. The use of data recorded within the first year after duAGPs was a subjective decision, being a compromise between the amount of data available for estimation of the effect and the validity of the assumption about constant exposure to management factors.
Bias and representativeness
The sampling frame (veterinarians and farms) of the study population was restricted. The reason for this restriction was to enhance the feasibility of the data collection procedure, i.e. the veterinarians and pig producers had to have motivation for participating and recording treatment. A general drawback of the restriction is that the study population may not be representative of all Danish pig farms. However, we assume that the study farms were representative of well-managed farms having good cooperation with their veterinarian because both the pig producer and the veterinarian displayed sufficient motivation in accepting the extra workload to complete the questionnaires and record treatments. In this type of farms, the effect of duAGPs could be less than in less efficiently managed farms. Therefore, the study may underestimate the effect of duAGPs in Danish pig production.
Further, the total number of veterinarians in Denmark that specialize in pig production is around 120, so at the veterinarian level the study population included ~10%. However, not all pig farms in Denmark are serviced by veterinarians specializing in pig production, and the study population may be biased towards pig producers advised by veterinarians specializing in pig production. We assumed the possibility of substantial selection bias at the farm level, regarding the effect of duAGPs, was low because the sampling of farms was done prior to discontinuation.
When comparing farrow-to-finish farms (producing half of all pigs for slaughter in Denmark) and farms specializing in production of finishers (producing the other half of pigs for slaughter in Denmark), the later type of farms seemed to have more aggravated problems with diarrhoea. One explanation could be that they allow the mingling of pigs they receive from different sources. We believe that the effect of duAGPs in farms that deliver pigs to specialized finishing farms, and within the finishing farms, must as a minimum have been the same as the effect observed in farrow-to-finish farms.
Even though only half of the initial 134 farms enrolled in the study were used in the statistical analysis, there was no indication that the dropout was non-random. For none of the response variables, was there a statistically significant difference between the 68 farms included in the analysis and the 38 farms excluded owing to a too short study period after duAGPs. Statistical comparison of the distribution of management factors in the 68 farms included in the data analysis and the distribution of management factors in the farms that returned the questionnaire (but were not included because of different reasons), did not reveal any differences in management between the included and excluded farms.
In all study farms, the use of AGPs to finishers was discontinued before the study start, and an eventual long-lasting effect of duAGPs in finishers might cause confounding in the estimation of the effect of duAGPs in weaners. However, because the time elapsed from the date of duAGPs for finishers until the date of duAGPs for weaners in all farms was at least 1 year in all study farms (ranging from 1 to almost 3 years, with a mean of 1·5 years) and because there was no correlation between the date for duAGPs to finishers and the date for duAGPs to weaners (Spearman correlation coefficient=0·08, P=0·49), we do not consider a potential long-lasting effect of duAGPs in finishers to be a strong confounding factor in our study.
Validity of recorded data
All pig producers probably did not record antibiotic treatment with the same degree of precision. This may be due to lack of time, capability or care. However, under the assumption that the within-farm precision was constant throughout the study period, due to the case-crossover design the between-farm variation in precision of recording did not influence the estimates.
Statistic analysis
The effect of duAGPs was evaluated for multiple diseases – in separate analyses – giving a relatively large probability (1–0·955) of declaring a non-true effect of duAGPs on the risk of disease significance. Nevertheless, we were more worried about not identifying existing effects than the reverse. Therefore, a confidence level of 95% was used in the analysis of each disease to preserve the power of the study.
To model the hierarchical structure of collected data, we specified multilevel models, including both random intercept and random slope. The REML procedure that we used for estimating parameters in the linear multilevel models is well established. However, procedures for fitting logistic multilevel models are not well established. We used two alternative estimation methods to obtain revised estimates of the final models – MCMC and ML. If reasonable concordance among the results is achieved – which was the case in our study – then one can have confidence in the results [Reference Rasbash13].
The correlation of PPT diarrhoea within farms between two adjacent days where treatment for diarrhoea was performed was high (correlation coefficient=0·71). Within the multilevel logistic models of the PDT diarrhoea, independence between observations was assumed. To obtain a crude estimation of the correlation of the risk for treatment between two adjacent days a model equivalent to the linear model used for PPT, was fitted to the binary dependent variable. Regarding the risk for treatment, the within-farm correlation between two adjacent days was 0·42. This correlation indicates that PDT diarrhoea between adjacent days within the same farm was not totally independent. However, given the moderate size of the correlation, together with other aspects of the healthy behaviour of the model, we are convinced that the significance of the estimated effect of duAGPs on PDT was related to a true effect.
Effect of discontinued use of antimicrobial growth promoters on risk of antibiotic treatment
The discontinuation only had a significant effect on the risk of treatment for diarrhoea (Tables 5 and 6). For the risk of treatment for other diseases, neither the estimated value of the effect nor the statistical significance indicated even a small effect on the frequency of arthritis, pneumonia, unthriving or miscellaneous disorders. On average, the daily risk that treatment was performed for diarrhoea (PDT diarrhoea) increased by a factor 2·5 (OR 2·5, 95% CI 1·7–3·9), whereas, on average, the risk for a pig achieving treatment at days where treatment was performed (PPT diarrhoea) increased by a factor of 1·6 (OR 1·6, 95% CI 1·1–2·2). The product of the two average estimates is approximately equal to four, which can be interpreted as the total effect of the discontinuation on the risk of treatment for diarrhoea. The increased risk of antibiotic treatment for diarrhoea in pigs after withdrawal of AGPs from weaners is also reflected in the total usage of antibiotics in 1998–2002 presented by the danmap programme [Reference Heuer and Larsen14]. When comparing the usage of kg active compounds in 1998 (AGPs were used in weaners, but not in finishers) with that of 2001 (no use of AGPs in any pig production), the use of tetracycline increased by a factor of 2·4 and the use of macrolides, lincosamides and tiamulin increased by a factor of 2·8. This increase was too large to be explained by an increase in animal production during the same period. In Denmark, these types of antibiotics are frequently used for diarrhoea in pig production. In accordance to statistics of prescribed antibiotics by veterinarians, which indicate that almost all usage of these antibiotics is related to pigs [Reference Heuer and Larsen14], the findings in our study indicate that the increased nationwide usage of antibiotics for treatment in food animals in Denmark in the period after the use of AGPs was stopped was a result of an increased frequency of antibiotic treatment for diarrhoea in pigs.
Prior to performing the data analysis, we expected an effect of the type of AGP used prior to discontinued use, because of differences in the antibacterial spectrum of AGPs. This effect could not be verified in our study.
Several agents have been suggested as possible causes of diarrhoea in growing pigs. In the Danish pig production, Lawsonia intracellularis, Brachyspira hyodysenteriae, Brachyspira pilosicoli [Reference Møller15] and pathogenic Escherichia coli [Reference Møller15, Reference Svensmark16] have been identified as important intestinal agents causing diarrhoea in growing pigs.
The AGPs used in pigs, are active against several of the listed pathogens. It has been proved that avilamycin, at twice the dose used as AGPs in Denmark, effectively prevents post-weaning diarrhoea in pigs [Reference Kyriakis17]. Moreover, the preventive effect of carbadox on post-weaning diarrhoea is well known. Regarding the preventive effect against spirochaete-related diarrhoea in finishers, it is documented that in-feed administration of carbadox (50 ppm) [Reference De Graaf18] has a preventive effect. The diarrhoea-preventing effect of tylosin is mainly related to the activity of tylosin against L. intracellularis. In a challenge study, in-feed administration of 40 ppm tylosin followed by 20 ppm tylosin effectively prevented proliferative enteropathy due to L. intracellularis [Reference McOrist19]. Furthermore, recent studies of the aetiology of mild diarrhoea in finishers [Reference Johnston20] indicate that mild diarrhoea can be attributed unknown pathogens, which may be prevented by the use of AGPs. As evident in the Danish pig production, AGPs were administered in doses approximating the doses proved to prevent various intestinal infections in pigs.
The significant time-dependent effect of duAGPs on PDT diarrhoea – with the effect of duAGPs not being significant during the first 3 months after discontinuation – was probably related to a carry-over effect caused by the previous use of AGPs. In addition, the potential infectious pressure of intestinal pathogens within the farms probably increased slowly.
However, no significant effect of time passing on PPT diarrhoea was identified, which means that the proportion of pigs treated with antibiotics at days when antibiotics were used increased immediately after the withdrawal. The immediate effect does not fit the assumption of carry-over effect and a slow increase in the infectious pressure. A possible change in the pig producers' and veterinarians' treatment policy overnight can explain the immediate effect – hereby possibly being an effect of a psychological nature.
As indicated by the large between-farm variation in the effect of duAGPs on PDT diarrhoea and PPT diarrhoea, an important part of the risk for increased occurrence of treatments of diseases can be related to factors that vary between farms. The low degree of correlation between the PDT diarrhoea before duAGPs (intercept) and effect of duAGPs indicated that both farms that performed treatment for diarrhoea frequently as well as not so frequently before duAGPs experienced problems with diarrhoea after duAGPs.
In our study we found no significant management interaction terms. However, because many different management factors influence the occurrence of diarrhoea, it can be assumed that the effect of duAGPs truly interacts with management factors at the farms. The lack of significant management interaction terms in our study is probably due to low power because the sample size was too small and not because there was truly no interaction.
Many experiments conducted with young piglets have shown that a dietary content of 2000–3000 ppm zinc oxide significantly reduces the incidence of diarrhoea. However, legislation until 2004, dictated that dietary levels above 250 ppm zinc were not allowed in pig diets. In our study, we could not find an interaction effect between using 250 ppm zinc in the pig diet and the effect of duAGPs.
With the exception of differences in management factors, a reasonable explanation for the variation among farms may be variation in the prevalence of L. intracellularis, B. hyodysenteriae, B. pilosicoli and pathogenic E. coli, which has been described in finisher pigs [Reference Stege21]. The same pattern of between-farm variation is probably also true for younger pigs. The observation that some pig producers experienced substantial problems with diarrhoea after cessation of AGPs, while others experienced few or no problems, may also be attributed to a biological range of variability. Finally, the treatment threshold may vary among pig producers, and this will contribute to the variation in the effect of duAGPs on the risk of antibiotic treatments, even if farms experience the same level of disease problems.
The pattern that some pig producers experienced substantial increase in the frequency of therapeutic antibiotic treatment for diarrhoea, while others experienced none or a small increase after withdrawal of AGPs can be expected within other countries of the European Union following the ban of AGPs. The effect will probably depend on the type of infectious diseases that are present in the populations and on the overall production facilities [Reference Casewell2].
How AGPs improve growth in pigs is not fully understood [Reference Gaskins, Collier and Anderson22]. Our study indicated that all antibiotics used as growth promoters had prophylactic activity against intestinal disorders and that withdrawal was associated with a general increased risk of treatment for diarrhoea in pigs. A similar effect was also observed in Sweden after duAGPs in pigs [Reference Wierup5]. The result of our study showed that within the first year after the use of AGPs was withdrawn, the pig producers used antibiotics to control diarrhoea in pigs.
In Table 7, we have summarized the amount of antibiotics prescribed to weaners and finishers in Denmark annually during the time period 2001–2004. According to the VetStat register there has been an annual increase in the crude amount of prescribed antibiotics to pigs (weaners and finishers) from 2001 to 2004. When comparing the amount of antibiotics prescribed annually per pig produced annually in two adjacent years, there is an annual increase in the amount of antibiotics prescribed for the disease groups arthritis and pneumonia – disease groups we found to be uninfluenced by duAGPs – whereas the amount prescribed for diarrhoea slightly decreased from 2001 to 2003. These tendencies, together with the result of our study, strongly indicate that the increase in treatment for diarrhoea caused by duAGPs was stabilized within one or a few years after AGPs were removed from production, and that the crude increase in amount of antibiotics prescribed to pigs from 2001 to 2004 in Denmark (the second to fifth year after duAGPs) is mainly caused by factors other than duAGPs.
Table 7. The amount of antibiotics prescribed to pigs annually (VetStatFootnote *) divided by the number of pigs slaughtered in Denmark annually. Amount per pig stratified by disease
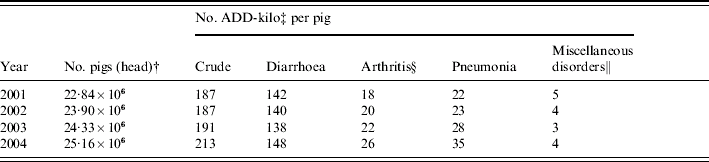
* In Denmark, all therapeutic drugs are prescription-only, and from 2001, the Danish register of veterinary medicines, VetStat, has collected detailed data on medicine consumption in animals [Reference Stege23].
† Number of pigs produced in Denmark [24].
‡ No. ADD-kilo (animal defined daily doses) – the no. kg pig that can be treated with the prescribed amount of antibiotics [Reference Jensen, Jacobsen and Bager25].
§ Includes also diseases in skin and central nervous system (i.e. not the same diseases groups as in the present project).
∥ Includes unthriving (i.e. not the same diseases groups as in the present project).
ACKNOWLEDGEMENTS
We thank the pig producers (owner and stable staffs) and the veterinarians involved in the data collection. This study was part of a Danish research effort to examine the effects of discontinued use of antimicrobial growth promoters in the Danish animal production. The research was financed by the Danish Ministry of Food, Agriculture and Fisheries.
DECLARATION OF INTEREST
None.














