INTRODUCTION
Necrotizing fasciitis (NF) is a rapidly progressing soft-tissue infection that represents a true medical and surgical emergency [Reference Young1]. Case-fatality rates for NF may exceed 30% and have remained high despite advances in the care of these patients [Reference Young1]. Persons with NF are almost uniformly managed in the hospital, often within a critical care unit, and frequently require repeated trips to the operating suite for control of the necrotizing process. The health-care and economic resources required to care for patients with NF are significant. The average cost to treat NF approaches US$50 000–100 000 per case [Reference Widjaja2, Reference Faucher3]. A better understanding of the factors associated with prolonged hospitalization, patient mortality, and the cost of care is required to identify potential targets for improving patient outcomes and optimizing health-care resource utilization.
Several studies have evaluated risk factors for prolonged length of stay (LOS) and mortality among patients hospitalized for NF [Reference Sudarsky4–Reference Tillou8] (Table 1). The majority of these have suffered from small sample sizes and have tended to be single-institution studies rather than population-based studies [Reference Tiu6–Reference Tillou8]. In addition, previous case series have not provided much information about the impact of hyperbaric oxygen (HBO) therapy, a treatment modality that remains a controversial adjunct to standard therapy for NF [Reference Young1, Reference Jallali, Withey and Butler9]. Because the use of HBO is associated with increased health-care costs [Reference Widjaja2], its impact on both LOS and mortality is important.
Table 1. Selected clinico-epidemiological studies of necrotizing soft-tissue infections (including necrotizing fasciitis) in which multivariate analyses were used

We conducted a relatively large retrospective cohort study to address the aforementioned limitations of earlier studies of the clinical epidemiology of NF. Our goals were to evaluate the impact of demographic and clinical factors, including HBO, on total patient charges (TC), LOS, and hospital mortality among patients hospitalized for NF during a 1-year period. Results from this study are intended to guide future, prospective investigations and identify factors that might aid clinicians in making therapeutic or prognostic decisions in the care of patients suffering from NF. To our knowledge, the use of a statewide hospital database represents a novel approach in the study of necrotizing soft-tissue infections.
METHODS
Source population and inclusion criteria
A hospital discharge dataset was obtained from the Florida Agency for Health Care Administration (AHCA). This public-use database includes discharge summaries from all non-federal Florida hospitals except state tuberculosis and state mental health hospitals. After data are entered into this system, they are subjected to formatting and logic checks. The primary hospital submitting patient information must then certify the data are correct and verify the accuracy of a summary report before it is released by the AHCA.
This dataset contained clinical and demographic information for 2 343 330 patients who were hospitalized for at least 1 day and discharged in calendar year 2001. The principal discharge diagnosis and up to nine secondary discharge diagnoses were coded using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Procedures performed during the hospital stay were also coded using ICD-9-CM. AHCA allowed for up to nine secondary procedure codes in addition to the principal procedure code.
Records were included in our analysis if the principal discharge diagnosis was NF (ICD-9-CM code 728.86). The records of patients who were discharged to another hospital were deleted to minimize the probability of including multiple records for a single patient in the multivariate analysis. After deleting records that had missing values for the dependent and/or independent variables, there were 216 records available for the statistical analysis.
Definition of outcomes
Three outcomes were studied. The first dependent variable was LOS recorded in days. According to AHCA, discharges on the admission date were counted as 1 day. The second dependent variable was also a continuous outcome, TC. This was defined by AHCA as the amount charged to the patient (before any discounts) and rounded to the nearest US dollar. The final outcome was hospital mortality.
Definition of potential risk factors
Several demographic and clinical risk factors were studied. Age at the time of admission was initially a continuous variable recorded in years but was eventually converted to a categorical variable because the risk of hospital mortality did not increase linearly with age in our dataset. The binomial regression model assumes that the logarithm of the probability of death increases or decreases smoothly in a linear fashion with the risk factor being studied. Examination of the risk profile indicated that age should be dichotomized at 44 years. The new binary variable compared patients who were aged ⩾44 years to those aged 0–43 years.
We created a binary race variable: white non- Hispanic vs. other race/ethnicity. The majority of the cases (94%) were whites of non-Hispanic ethnicity or African-American. Patient insurance payer was defined as commercial insurance compared to non-commercial insurance. Commercial carriers were defined as non-Medicaid health maintenance organizations and preferred provider organizations. Non-commercial carriers included Medicare, Medicaid, charity, self-pay, Veterans Affairs, etc. Diabetes was defined as the presence of ICD-9-CM code 250.0–250.9 in any of the nine secondary discharge diagnosis positions. A patient was considered to have group A streptococcal NF if ICD-9-CM code 041.01, group A Streptococcus, was found in any of the secondary discharge diagnosis fields. HBO therapy was defined as ICD-9-CM procedure code 93.59, hyperbaric oxygenation of wound, or ICD-9-CM code 93.95, hyperbaric oxygenation (respiratory therapy). Finally, we examined the impact of debridement which was defined as ICD-9-CM code 86.22 (excisional debridement of wound, infection, or burn) in any of the ten procedure fields.
Statistical analysis: LOS and TC
Exploratory analyses revealed that the distributions of LOS and TC for certain values of the independent variables were skewed. The residuals were also not normally distributed. Since LOS and TC were skewed variables and since patients were clustered in 87 hospitals, the use of conventional linear regression was not appropriate. To account for the lack of normality in the LOS distribution and TC distribution and the possibility of correlated outcome data we used robust gamma mixed regression [Reference Lee10]. The gamma distribution accommodates outliers while the combination of fixed and random effects defines a mixed model [Reference Lee10]. The seven risk factors listed above and a gender variable were treated as the fixed effects in our regression model while hospital was treated as the random effect. LOS was not included as a predictor while modelling correlates of TC since it is in the causal pathway of several of our predictor variables [Reference Szklo and Nieto11, Reference Rothman and Greenland12]. For example, HBO or surgery may lead to a prolonged LOS which would lead to a higher TC.
Robust gamma mixed regression was performed using the glimmix macro in SAS (SAS Institute Inc., Cary, NC, USA). A logarithmic link function was adopted to ensure the projection of a positive mean LOS and TC rather than nonsensical values of LOS and TC such as a zero or negative value. A result was considered statistically significant if the P value was ⩽0·05. Ninety-five percent confidence intervals (CI) were also calculated. Zero was the null value of no association.
Statistical analysis: hospital mortality
We performed binomial regression to identify risk factors for hospital mortality [Reference Robbins, Chao and Fonseca13]. In the setting of a cohort study, binomial regression yields relative risk (RR) estimates while logistic regression would yield incidence odds ratios. Crude and adjusted RRs for hospital mortality were calculated using proc genmod in SAS [Reference Robbins, Chao and Fonseca13]. An RR was considered statistically significant if the 95% CI for the population RR excluded the null value of 1.
Several of our independent variables may have been correlated so we calculated tolerances in an attempt to diagnose collinearity [Reference Allison14]. We did not detect any multicollinearity. As stated above, the assumption of independent observations was probably violated since patients were nested by hospital. Failure to adjust for this correlation may affect point estimates and P values. We used generalized estimating equations to account for potential correlations between patients treated at the same hospital [Reference Cole15]. An exchangeable correlation structure was specified [Reference Hosmer and Lemeshow16].
RESULTS
Our initial survey of the AHCA database identified 238 cases coded as NF at the time of discharge or death who had complete data on the variables of interest. Twenty-two of these cases were excluded from further analysis because the patients were discharged to another hospital and were at risk of being counted twice. Two hundred sixteen cases were included in subsequent analyses, yielding an annual period prevalence of NF of ∼9·2 cases/100 000 hospitalized patients. Of the 216 patients, 209 were Florida residents at the time of admission. Dividing the latter figure by the population of Florida in 2001 (16 410 669) produced an unadjusted hospitalization rate of 1·3 cases/100 000 population [17].
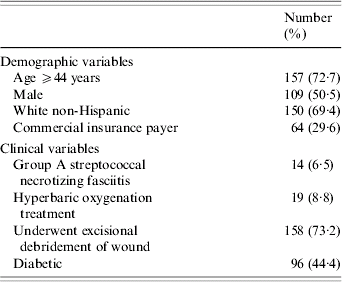
Selected characteristics of these 216 patients are shown in Table 2. A large proportion of the cases were aged ⩾44 years at the time of admission (72·7%). Group A Streptococcus was noted to be involved in only 14 cases (6·5%).
Table 2. Demographic and clinical characteristics of patients hospitalized for necrotizing fasciitis (n=216)
The distributions of LOS and TC were skewed to the right (data not shown). The median LOS was 13 days (minimum=1 day, maximum=172 days) while the median TC was $54 533 (minimum=$5760, maximum=$550 687). Cumulative TC for the entire cohort were $18 133 445.
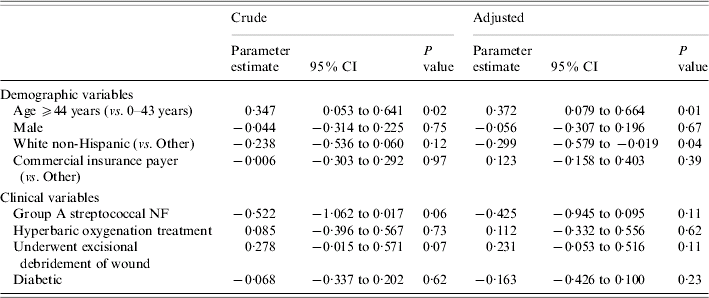
Crude and adjusted coefficients from the robust gamma mixed regression when LOS was the outcome are shown in Table 3. These parameter estimates cannot be interpreted as estimates from an ordinary linear regression model. For example, the parameter estimate for age ⩾44 years (unadjusted) is 0·347. In an ordinary linear regression, one could conclude that older age was associated with an increase in the LOS of 0·347 days. This interpretation is not possible in our analysis since a logarithmic link was used (see Statistical analysis section above). As presented, the effects of the independent variables can only be interpreted on the log-scale; however the estimates in Table 3 do quantify the magnitude and direction of the association. In both the crude and adjusted analyses age ⩾44 years was significantly associated with a prolonged LOS. After adjusting for age, sex, and the remaining five variables, white non-Hispanics had a shorter LOS than patients of other races/ethnicities (P=0·04). Surgical debridement, a marker of disease severity, was not associated with LOS (adjusted parameter estimate=0·231, P=0·11).
Table 3. Crude and adjustedFootnote * parameter estimates of potential risk factors for length of stay from fitting robust gamma mixed regression models
NF, Necrotizing fasciitis; CI, confidence interval.
* Each parameter estimate is adjusted for the remaining variables in the table.
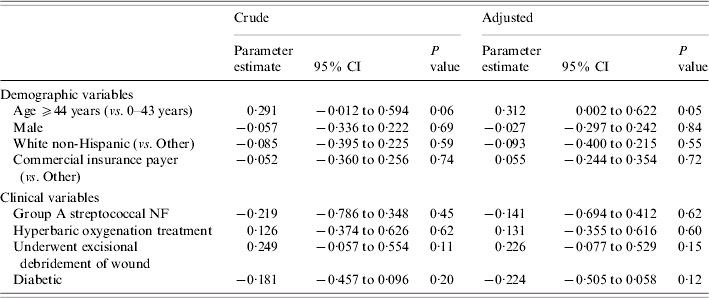
Table 4 presents coefficients from the robust gamma mixed regression models where TC was the outcome. Older age (⩾44 years) was not associated with TC in the unadjusted analysis (P=0·06), but after adjusting for the remaining seven variables shown in Table 4, patients who were aged ⩾44 years were more likely to have elevated TC compared to patients who were aged 0–43 years (P=0·05). The impact of surgical debridement on TC did not change appreciably after adjusting for the covariates.
Table 4. Crude and adjustedFootnote * parameter estimates of potential risk factors for total patient charges from fitting robust gamma mixed regression models
NF, Necrotizing fasciitis; CI, confidence interval.
* Each parameter estimate is adjusted for the remaining variables in the table.
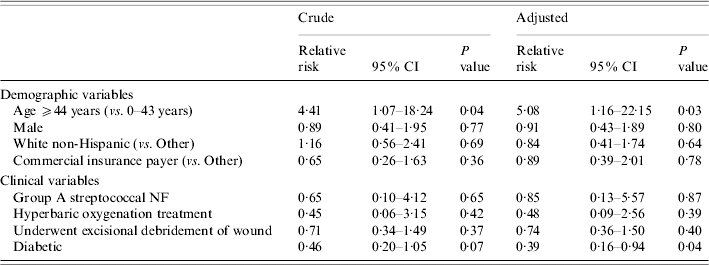
The crude mortality rate was 11·1% (24/216). Examining hospital mortality by quartiles of age revealed that the risk of death increased sharply between the first two age groups but remained approximately constant thereafter (Fig.). Crude and adjusted RRs for hospital mortality are shown in Table 5. Patients in the older age group were five times as likely to die in the hospital as younger patients (adjusted RR 5·08, P=0·03). Diabetics were 61% less likely than non-diabetics to die in the hospital (adjusted RR 0·39, P=0·04). This protective RR is adjusted for any confounding effects of surgical debridement, age, and the other variables shown in Table 5.

Fig. Mortality rate of hospitalized patients suffering from necrotizing fasciitis. The percent of patients who died while hospitalized with necrotizing fasciitis during the 2001 calendar year are shown as a function of age.
Table 5. Crude and adjustedFootnote * relative risks for hospital mortality from binomial regression models using generalized estimating equations
NF, Necrotizing fasciitis; CI, confidence interval.
* Each relative risk is adjusted for the remaining variables in the table.
The AHCA database did not have information on the time between hospital admission and the performance of any of the nine secondary procedures. However, this dataset did contain information on the number of days that had elapsed between admission and the performance of the principal procedure (the procedure that was coded in the first of the ten procedure fields). Of the 158 patients who underwent surgical debridement in our study, 135 had this procedure (ICD-9-CM code 86.22) listed in their principal procedure field. Among these 135 patients, 51 patients received debridement on the day of admission. The crude hospital mortality rate among those who did not receive debridement on the day of admission was 11·9% while the mortality rate among those who were operated on on the day of admission was 5·9% (Two-tailed Fisher's exact test P=0·37). Due to the small number of patients (n=3) who both underwent debridement on the day of admission and died we could not perform a multivariate analysis of the association between the timing of debridement and hospital mortality.
We considered the possibility that patients who were admitted for NF who did not undergo debridement may have died rapidly, thereby precluding surgical exploration and the removal of devitalized tissue. However, among the 58 patients who did not receive debridement, the median LOS was 10 days, suggesting that this was not the case.
DISCUSSION
NF is a serious infection that has rarely been studied using population-based data. The current analysis of a statewide database attempted to identify factors associated with LOS, TC, and mortality among patients hospitalized for NF in Florida.
The crude hospital mortality rate in our case series was 11·1%, which is lower than that reported in other contemporary studies of NF [Reference Wong7, Reference Childers18]. Whereas this discrepancy might result from the inclusion of patients with less severe fasciitis or diagnostic misclassification, our data accord with a report by Schnall et al. who noted an 11% mortality rate among 99 cases of NF treated at their institution in Los Angeles, California, between 1993 and 1995 [Reference Schnall19] and with Kao et al. who reported an overall mortality rate of 12% in a study of 59 patients treated between 1995 and 2002 [Reference Kao20]. The overall mortality in our cohort may also have been lower than other published reports because of the fewer number of group A Streptococcus-associated cases. The mortality of NF is generally higher when group A Streptococcus is involved [Reference Young1]. It is possible that we may have underestimated the number of cases involving group A Streptococcus due to diagnostic code misclassification. When we broadened the ICD-9-CM code for group A Streptococcus (041.01) to include any streptococcal species (codes 041.00–041.09), we identified an additional 20 cases. However, the presence of any streptococcal species was not associated with death in a limited multivariate analysis (data not shown).
We detected an increasing risk of hospital mortality with advancing age, a finding that has been reported before [Reference Kaul5]. Schnall et al. divided their case series into two age groups using 40 years of age as the dividing point. They found that the older group was 9·7 times as likely to die than the younger group (27% compared to 2·8%) [Reference Schnall19].
We found that after adjusting for seven demographic and clinical variables, diabetes mellitus remained strongly associated with survival (adjusted RR of mortality=0·39, P=0·04). This counterintuitive result merits further study, although potential explanations can be postulated. Because diabetes mellitus is a well-known risk factor for necrotizing soft-tissue infections [Reference Young1], it is quite possible that diabetic patients were suspected of having NF at an earlier stage of illness than non-diabetic individuals. If such a lead time bias existed it would be expected to result in more rapid diagnosis and treatment of NF in diabetic patients, possibly reducing their morbidity and mortality compared to the rest of the cohort. In addition, it has been reported that chronic conditions such as diabetes may not be recorded in administrative health-care databases for patients who die in the hospital since the secondary diagnosis fields are taken up by acute conditions such as respiratory and/or cardiac arrest [Reference Iezzoni21]. This bias against coding chronic conditions on the computerized discharge abstract of patients who die would make diabetes appear to be associated with a lower risk of in-hospital death [Reference Iezzoni21]. Although we cannot rule out a similar effect in our study, it is relevant that none of the 24 patients who died and none of the 192 patients who were discharged had the ICD-9-CM code for respiratory arrest (799.1) in any of their nine secondary diagnosis coding spaces. Only two of the 24 patients (8·3%) who died in-hospital had a code for cardiac arrest (427.5) in their record. Furthermore, several studies of NF have failed to find a higher mortality rate for diabetics than for non-diabetics (see [Reference Kao20] and the references cited within). In our previous study of 195 patients hospitalized for invasive group A streptococcal disease, of whom 17% had NF, we discovered that diabetic status was not associated with hospital mortality (adjusted odds ratio=0·62, 95% CI 0·17–2·18) [Reference Mulla, Leaverton and Wiersma22].
Our analysis revealed that HBO was not associated with increased TC, a result that was surprising given its expense [Reference Widjaja2]. Nor did we observe an improvement in survival with HBO before or after adjustment for covariates. These results are difficult to interpret in large part due to the small number of patients who received HBO treatment (n=19) and the small number of deaths overall.
It is notable that the patient charges in our study (median $54 533) were quite similar to other recent estimates of the expenses involved in caring for patients with NF [Reference Widjaja2, Reference Faucher3]. That the annual TC for all 216 NF patients were over $18 million underscores the importance of identifying determinants of cost that might represent targets for optimizing health-care resource utilization. It should be emphasized, however, that these estimates do not take into account either the costs of post-discharge physical rehabilitation for NF patients or the indirect expense to the community in lost productivity and workforce reduction.
Our economic analyses are limited by the use of hospital charges as opposed to actual costs. Although hospital charges are not reliable measures of cost, such figures serve as a surrogate cost indicator, allowing a crude assessment of the relative economic burden of a particular diagnosis. For example, the magnitude of the TC for NF can be contrasted with TC of $7925 associated with a principal discharge diagnosis of cellulitis (ICD-9-CM code 682.9, n=31 patients) calculated from the same database (data not shown). This difference in charges probably relates to the greater number of hospital bed days, diagnostic tests and surgical interventions needed to care for patients with fasciitis, as opposed to cellulitis.
We proposed that the financial burden of NF might be significantly reduced by diagnosing the disease in its earliest stages. Clues to the presence of early NF can be gleaned from epidemiological risk factors (such as associated comorbidities), physical signs and symptoms, and/or specific laboratory abnormalities (reviewed in [Reference Young1]). Early, aggressive medical and surgical management of NF is predicted to reduce the number of bed days and surgical procedures required to control the spread of infection [Reference Sudarsky4], which would translate into reduced cost. An investment on the part of health-care facilities and providers to develop and institute guidelines or clinical care pathways for the evaluation and management of patients presenting with acute skin and soft-tissue complaints might help achieve this goal.
The present study has several strengths. First, by examining a large number of cases throughout the state of Florida, our approach limits the possibility of institutional bias. Second, the use of a state discharge database appears to be a novel approach to studying the outcomes of NF. A search of the PubMed/Medline database (Bethesda, MD, USA) did not reveal any similar studies that used a state administrative health-care database. Furthermore, we performed a comprehensive analysis of LOS using a multivariate technique. Four previous studies of NF simply reported the median or mean LOS with little or no elucidation of risk factors for prolonged LOS [Reference Sudarsky4, Reference Tiu6, Reference Tillou8, Reference Endorf, Supple and Gamelli23]. We accounted for any possible correlation in the development of the outcomes of interest among patients within a particular hospital by either fitting a random- effects parameter for the hospital identification code or using generalized estimating equations. Failure to account for this violation of independence would lead to erroneous associations. The use of robust gamma mixed regression also allowed us to avoid transforming the outcomes of LOS and total charges or arbitrarily deleting the outliers. This technique appears to be a suitable alternative to analysing data that are both skewed and clustered [Reference Lee10].
Our analysis had several limitations. Diagnostic misclassification could have biased our results by either failing to identify true NF cases (false negatives) or including patients incorrectly classified as having NF (false positives). Similar problems could have biased the other variables included in our analyses (e.g. diabetes mellitus or group A streptococcal infection). The sensitivity and specificity of the coding of principal and secondary discharge diagnoses in the AHCA database is unknown; however, as discussed in the Methods section, each reporting hospital must certify that their data are correct. The retrospective nature of our study prohibited any confirmation of the diagnosis of NF by conventional means (surgical inspection/histopathology). We were also unable to differentiate between community-acquired and nosocomial cases of NF, which may possess unique determinants of LOS and/or mortality.
Furthermore, the AHCA database did not have comprehensive data on the timing of surgery. The timing of surgery is an important determinant of survival. Wong et al. reported on the clinical epidemiology of 89 patients admitted for NF [Reference Wong7]. They found that a delay in surgery of greater than 24 h was correlated with increased mortality (RR=9·4, P<0·05). Information regarding the extent of debridement, which might also impact on TC and LOS, was also lacking in our study.
Another limitation of this investigation was the lack of information on the use of particular antibiotics during the hospital stay. For example, clindamycin has been shown to improve outcomes in the setting of deep infections, such as NF and myositis, caused by group A Streptococcus in both animal and epidemiological studies [Reference Mulla, Leaverton and Wiersma22, Reference Stevens24, Reference Zimbelman, Palmer and Todd25]. As our series included few documented group A streptococcal infections, this may not have been relevant. However, surgery remains the foundation of NF treatment [Reference Young1] and our database did contain information on surgical debridement.
In summary, this large, retrospective investigation of hospitalized NF patients documents that age ⩾44 years was the strongest correlate of prolonged LOS and elevated TC. Age ⩾44 years was also clearly the most powerful predictor of an increased risk of hospital mortality. As our population ages and the number of elderly increases, the problem of necrotizing soft-tissue infections may be expected to rise proportionally. Our results highlight the substantial economic toll imposed by caring for patients with NF and re-emphasize the need for continued studies aimed at the prevention and treatment of this devastating clinical syndrome.
ACKNOWLEDGEMENTS
D. M. Aronoff was funded by National Institutes of Health grant HL078727.
DECLARATION OF INTEREST
None.










