Introduction
Depression and obesity are highly comorbid disorders, rapidly increasing in prevalence globally (Finkelstein, Ruhm, & Kosa, Reference Finkelstein, Ruhm and Kosa2005; Otte et al., Reference Otte, Gold, Penninx, Pariante, Etkin, Fava and Schatzberg2016). The co-occurrence of depression and obesity are leading causes of disability and mortality (DiMatteo, Lepper, & Croghan, Reference DiMatteo, Lepper and Croghan2000; Milaneschi, Lamers, Berk, & Penninx, Reference Milaneschi, Lamers, Berk and Penninx2020; Strawbridge et al., Reference Strawbridge, Arnone, Danese, Papadopoulos, Herane Vives and Cleare2015; Upadhyay, Farr, Perakakis, Ghaly, & Mantzoros, Reference Upadhyay, Farr, Perakakis, Ghaly and Mantzoros2018), which presents a significant healthcare concern. The association between depression and obesity appears stronger in longitudinal studies than in cross-sectional studies (Luppino et al., Reference Luppino, de Wit, Bouvy, Stijnen, Cuijpers, Penninx and Zitman2010; Milaneschi et al., Reference Milaneschi, Lamers, Peyrot, Baune, Breen and Dehghan2017), suggesting that a common pathophysiological mechanism is driving this high comorbidity. Further highlighting the bidirectional relationship between depression and obesity, weight loss interventions are associated with improvements in depressive symptoms (Capuron et al., Reference Capuron, Poitou, Machaux-Tholliez, Frochot, Bouillot, Basdevant and Clement2011; Perez-Cornago et al., Reference Perez-Cornago, de la Iglesia, Lopez-Legarrea, Abete, Navas-Carretero, Lacunza and Zulet2014).
The only effective, long-term weight loss intervention for obesity is bariatric surgery, where patients can lose up to 60% to 70% of their excess weight (Schroeder, Garrison, & Johnson, Reference Schroeder, Garrison and Johnson2011). Although the rapid weight loss induced by bariatric surgery is typically associated with a dramatic improvement in depressive symptoms (Fu, Zhang, Yu, Mao, & Su, Reference Fu, Zhang, Yu, Mao and Su2021; Gill et al., Reference Gill, Kang, Lee, Rosenblat, Brietzke, Zuckerman and McIntyre2019; Spirou, Raman, & Smith, Reference Spirou, Raman and Smith2020), some studies have observed that a preoperative depression diagnosis is associated with reduced weight loss and worse depression prognosis following bariatric surgery (Galioto & Crowther, Reference Galioto and Crowther2013; Martens et al., Reference Martens, Hamann, Miller-Matero, Miller, Bonham, Ghaferi and Carlin2021; Pedro et al., Reference Pedro, Neves, Ferreira, Guerreiro, Salazar, Viana and Carvalho2020; van Hout, Verschure, & van Heck, Reference van Hout, Verschure and van Heck2005). However, the inconsistent findings may also be due to these studies using self-report questionnaires instead of clinical interviews to assess depressive symptoms (Spirou et al., Reference Spirou, Raman and Smith2020). Given that depression rates are very high in bariatric surgery candidates, with approximately 45% of bariatric surgery having a presurgical diagnosis of depression (Alabi et al., Reference Alabi, Guilbert, Villalobos, Mendoza, Hinojosa, Melgarejo and Zerrweck2018), there is an urgent medical need to identify whether depression is a risk factor for poor clinical outcomes after bariatric surgery (Van den Eynde et al., Reference Van den Eynde, Mertens, Vangoitsenhoven, Meulemans, Matthys, Deleus and Van der Schueren2021).
Chronic inflammation is a hallmark of obesity, as adipose tissue accumulation stimulates the production of proinflammatory cytokines, resulting in elevated C-reactive protein (CRP) levels (Thompson et al., Reference Thompson, Koehler, Herring, Paynter, Du, Zhang and Gordon-Larsen2016). Peripheral inflammation, driven by proinflammatory cytokine activity, has also been suggested to play a role in the pathophysiology of depression (Capuron & Miller, Reference Capuron and Miller2011; Dantzer & Walker, Reference Dantzer and Walker2014; Raison et al., Reference Raison, Dantzer, Kelley, Lawson, Woolwine, Vogt and Miller2010). Cytokine abnormalities in the periphery are closely associated with cytokine abnormalities in the central nervous system (CNS) (Enache, Pariante, & Mondelli, Reference Enache, Pariante and Mondelli2019), which can lead to neuroinflammation in brain regions such as the anterior cingulate cortex, hypothalamus, amygdala, and hippocampus (Borsini et al., Reference Borsini, Cattaneo, Malpighi, Thuret, Harrison, Consortium and Pariante2018; Duan et al., Reference Duan, Zeng, Zheng, Song, Li, Kong and Xu2018; Goldsmith, Bekhbat, Mehta, & Felger, Reference Goldsmith, Bekhbat, Mehta and Felger2022; Meier et al., Reference Meier, Drevets, Wurfel, Ford, Morris, Victor and Savitz2016; Zunszain et al., Reference Zunszain, Anacker, Cattaneo, Choudhury, Musaelyan, Myint and Pariante2012). Abnormalities within these brain regions and neurotransmitter systems are found in depression and obesity (Capuron, Lasselin, & Castanon, Reference Capuron, Lasselin and Castanon2017). Therefore, increased inflammation, primarily mediated by cytokines in the periphery and CNS, demonstrates a biological pathway that may underlie the high comorbidity between depression and obesity (Chan, Cathomas, & Russo, Reference Chan, Cathomas and Russo2019).
Given the putative role of inflammation in the vicious cycle between depression and obesity, inflammation may influence weight loss and depression outcomes following bariatric surgery. Indeed, bariatric patients who exhibit more considerable reductions in inflammatory markers during the early post-surgical period demonstrate greater weight loss and improved metabolic health (Illán Gómez et al., Reference Illán Gómez, Gonzálvez Ortega, Aragón Alonso, Orea Soler, Alcaraz Tafalla, Pérez Paredes and Lozano Almela2016; O'Rourke et al., Reference O'Rourke, Johnson, Purnell, Courcoulas, Dakin, Garcia and Wolfe2019). Other studies have shown that reductions in BMI following surgery were associated with improved depressive symptoms and lower CRP levels (Capuron et al., Reference Capuron, Poitou, Machaux-Tholliez, Frochot, Bouillot, Basdevant and Clement2011; Perez-Cornago et al., Reference Perez-Cornago, de la Iglesia, Lopez-Legarrea, Abete, Navas-Carretero, Lacunza and Zulet2014). More recently, transcriptomic analyses of bariatric patients observed upregulated cytokine signaling pathways associated with depression severity at baseline and remission after surgery (Moisan et al., Reference Moisan, Foury, Dexpert, Cole, Beau, Forestier and Capuron2021). Disentangling the temporal relationships between changes in depressive symptoms and weight loss during the postoperative period is crucial, as longitudinal studies of bariatric patients suggest that changes in depressive symptoms and weight loss in the first postoperative year significantly influence long-term outcomes (Geerts et al., Reference Geerts, Van Den Berg, Van Riel, Peen, Goudriaan and Dekker2021; Smith et al., Reference Smith, Mason, Cao, Crosby, Steffen, Garcia and Mitchell2020). Together, these findings suggest that changes in inflammatory markers associated with depression may contribute to the high heterogeneity in weight loss and mental health outcomes among bariatric patients.
Here, to our knowledge, we conducted the first study to investigate differences in serum and adipose cytokines between obese patients with and without depression undergoing bariatric surgery. We aimed to identify risk factors for poor clinical outcomes after surgery by measuring inflammatory biomarkers and depression assessed by clinical interviews before surgery and 6 months after. We also assessed potential confounders, including socio-demographic variables, diabetic status, antidepressant use, childhood trauma, and physical activity. We hypothesized that: (1) bariatric patients with depression will demonstrate higher inflammation relative to control bariatric patients; (2) higher inflammation at baseline will predict reduced weight loss and higher severity of depressive symptoms after surgery.
Methods and materials
Study design
This longitudinal observational case–control study recruited patients undergoing bariatric surgery at King's College Hospital as part of the ongoing Bariatric Surgery & Depression (BARIDEP) study. We recruited 85 bariatric candidates before their bariatric surgery procedure and followed them up 6 months after, between March 2018 and November 2021. Patients provided written informed consent, underwent eligibility screening, and were compensated with vouchers for participation. We provided the vouchers after each study visit to reimburse patients for their time, but the voucher amounts were minimal to prevent the risk of financial incentives influencing their decision to participate in the study. The Ethics Committee of the National Research Ethics Service East of England approved the study (18/LO/350). We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for case–control studies and collected biological samples in accordance with the 2004 Human Tissue Act.
Participants
We collected socio-demographic, medical history, and neuropsychiatric data to assess patients for eligibility through structured clinical interviews with a trained researcher. Self-reported responses from patients were cross-validated with their medical records.
Participants were eligible if they were (1) obese, classified as BMI ≥ 35; (2) undergoing either Roux-en-Y Gastric Bypass (RYGB) or Sleeve Gastrectomy (SVG) bariatric surgery at King's College Hospital; (3) aged between 18 and 70 years old; (4) able to read and write English fluently. Participants were excluded if they (1) were pregnant or breastfeeding; (2) had a past or current primary diagnosis of psychotic disorder; (3) were taking high doses of anti-inflammatory medication likely to compromise the interpretation of immunological data (including, but not limited to, non-steroidal anti-inflammatory drugs for chronic pain, antibiotics, oral steroids, immunosuppressive drugs, or monoclonal antibodies that block inflammatory cytokines; see medication exceptions in page one of the Supplement); (4) met criteria for alcohol abuse, drug abuse or dependence in the last 6 months; (5) participated in a clinical trial of an investigational drug within the last year; (6) had a lifetime history of any severe medical disorder or recent infection or illness likely to compromise the interpretation of immunological data.
Patients with depression (cases) were eligible if they met the criteria for current major depressive disorder (MDD), as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), using the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998). Patients with depression were excluded if they had a lifetime history of bipolar disorder or non-affective psychosis. Control patients were eligible if they had no personal history of MDD or treatment with any antidepressant for depressive symptoms and no current or lifetime history of severe mental illness, including affective and psychotic disorders, as defined by the DSM-5, using the MINI.
Assessment of clinical measures related to depression and obesity
Depressive symptoms were measured using the Structured Interview Guide for the Hamilton Depression Rating Scale with Atypical Depression Supplement (SIGH-ADS), which included the 17-item Hamilton Rating Scale for Depression (HAM-D) (Hamilton, Reference Hamilton1960), and the National Institute of Mental Health addition of eight atypical depression symptoms (Singh & Williams, Reference Singh and Williams2006).
We also assessed other clinical variables associated with MDD and obesity in bariatric populations to control for potential confounders, including (1) the Childhood Trauma Questionnaire measured the severity of different types of childhood trauma, encompassing five clinical subscales: Emotional Abuse, Physical Abuse, Sexual Abuse, Emotional Neglect, Physical Neglect (Bernstein et al., Reference Bernstein, Fink, Handelsman, Foote, Lovejoy, Wenzel and Ruggiero1994); (2) the short-form International Physical Activity Questionnaire (IPAQ) assessed the physical activity levels of participants (Craig et al., Reference Craig, Marshall, SjÖStrÖM, Bauman, Booth, Ainsworth and Oja2003).
Body composition and weight loss
BMI and weight were measured on a Tanita MC-780MA professional body composition analyzer. Outcome weight loss was calculated as weight loss percentage (WLP), which is considered the most sensitive measure of postoperative weight loss (Hatoum & Kaplan, Reference Hatoum and Kaplan2013). We also reported excess weight loss percentage to allow for comparison with other studies (see online Supplementary Table S1 for equations).
Serum inflammatory markers
Markers of peripheral inflammation, measured in serum, included high-sensitivity CRP (hsCRP), proinflammatory cytokines IL-6, IL-2, TNF-α, IL-8, IL-1β, IL-12p70, IL-13, and anti-inflammatory cytokines IL-4 and IL-10. hsCRP is considered a reliable biomarker of low-grade chronic inflammation present in individuals with MDD, as hsCRP tests can detect slight elevations in CRP levels (between 0.5 to 10 mg/L) that would go unnoticed with standard CRP testing (that only detect acute inflammation above 10 mg/L). Blood samples were collected from participants at baseline and follow-up in the phlebotomy clinic at King's College Hospital; serum hsCRP samples were analyzed by Viapath laboratory. We measured serum cytokine levels using Meso Scale Discovery (MSD) V-PLEX sandwich immunoassays, MSD Pro-inflammatory Panel 1 human kit (Dabitao, Margolick, Lopez, & Bream, Reference Dabitao, Margolick, Lopez and Bream2011; King et al., Reference King, O'Brien, Donaghy, Williams-Gray, Lawson, Morris and Thomas2019a), and plates were read on an MSD QuickPlex SQ 120 (Hepgul et al., Reference Hepgul, Pariante, Dipasquale, DiForti, Taylor, Marques and Mondelli2012; Russell et al., Reference Russell, Hepgul, Nikkheslat, Borsini, Zajkowska, Moll and Pariante2019). All inter and intra-assay coefficients of variations were <10%. The lowest detectable values of cytokine assays are reported in online Supplementary Table S2. Clinically elevated hsCRP levels were defined as hsCRP ≥ 3 mg/L (Miller & Raison, Reference Miller and Raison2016; Pearson et al., Reference Pearson, Mensah, Alexander, Anderson, Cannon, Criqui and Vinicor2003).
Adipose inflammatory markers
Adipose tissue samples were collected during participants' bariatric surgery operations at King's College Hospital. Cytokines, measured in visceral adipose tissue, included proinflammatory IL-6, IL-2, TNF-α, IL-8, IL-1β, IL-12p70, IL-13, and anti-inflammatory IL-4 and IL-10. We measured cytokine levels using MSD V-PLEX sandwich immunoassays, MSD Proinflammatory Panel 1 human kit (Dabitao et al., Reference Dabitao, Margolick, Lopez and Bream2011; King et al., Reference King, O'Brien, Donaghy, Williams-Gray, Lawson, Morris and Thomas2019a), and plates were read on an MSD QuickPlex SQ 120 (du Plessis et al., Reference du Plessis, Korf, van Pelt, Windmolders, Vander Elst, Verrijken and van der Merwe2016; Gletsu et al., Reference Gletsu, Lin, Zhu, Khaitan, Ramshaw, Farmer and Smith2006). All inter and intra-assay coefficients of variations were <10%. Protein content in tissue homogenates was measured spectrophotometrically by protein OD A280 (NanoDrop, Thermo Fisher Scientific). Cytokine concentrations were normalized and expressed per milligram of protein present in adipose tissue to account for inter-individual fat cell size variability (DiGirolamo & Fine, Reference DiGirolamo, Fine and Ailhaud2001).
Statistical analysis
We conducted a power size calculation in G Power to determine a sample size requirement of >70 bariatric patients to have an 80% power of detecting an effect size of d = 0.68, alpha level = 0.05, p < 0.05 two-tailed, according to a meta-analysis finding higher IL-6 levels in depressed patients compared with healthy controls with an effect size of d = 0.68 [7].
Primary outcomes were evaluated in cases v. controls using analysis of covariance (ANCOVA). Inflammatory markers were logarithmic or square-root transformed. Covariates at baseline and time of surgery included age, BMI, gender, ethnicity, diabetic status, antidepressant use, and smoking status. Across the total sample, participants self-identified as African (5.9%), Asian (1.2%), Black British (8.2%), Caribbean (7.1%), Gypsy (1.2%), Hispanic (1.2%), Latin American (1.2%), multiracial (Black and White) (4.7%), other multiracial (2.4%), White British (63.6%), and White other (3.5%). Due to the large proportion of participants of white ethnicity and the small cell sizes of participants from other ethnic backgrounds, data on ethnicity were collapsed into a dichotomized variable where participants were classified as either being of white ethnicity or non-white ethnicity.
Sensitivity analyses showed no significant effect on gender, ethnicity, diabetic status, and time to follow-up on inflammatory markers at follow-up. Therefore, covariates for inflammatory markers at follow-up included participant group, age, BMI at follow-up, surgery type, smoking status since surgery, and antidepressant use after surgery. Further analysis of inflammatory markers showing significant group differences was carried out with sample-wide correlations between serum and adipose inflammatory markers, and between inflammatory markers with depression scores at baseline or follow-up.
Predictors of secondary outcomes (WLP and HAM-D scores at follow-up) were evaluated in the total sample using hierarchical regression modeling. Firstly, baseline covariates for models were evaluated in scatter plots and selected if they showed significant bivariate correlations with outcome measures. Hierarchical regression was carried out in steps: (1) strength and significance of socio-demographic and clinical confounders were assessed; (2) strength and significance of baseline predictors were assessed, controlling for confounders; (3) backward elimination removed weak predictors until the models with the highest performance metrics were identified for each outcome measure.
A two-tailed p value <0.05 was considered statistically significant. Statistical analyses were conducted by A.P.M. using R software, version 4.1.1.
Results
The sample included 85 bariatric surgery candidates with a mean (s.d.) age of 46.5 (11.6) years, and 51 (60%) participants were female. Before bariatric surgery, 41 patients with depression and 44 control patients completed a baseline assessment; 29 patients with depression and 37 control patients completed a follow-up assessment 6 months after bariatric surgery. Of the 29 patients who met the criteria for depression at baseline, 19 (65.5%) no longer met the clinical criteria for depression at their follow-up assessment. There were no significant differences in weight loss between groups at follow-up after adjustment for confounders. Socio-demographic, weight loss and clinical data are reported in Table 1. Childhood trauma scores are reported in online Supplementary Table S3.
Table 1. Sample characteristics, weight and psychopathology at baseline and 6-month follow-up after bariatric surgery
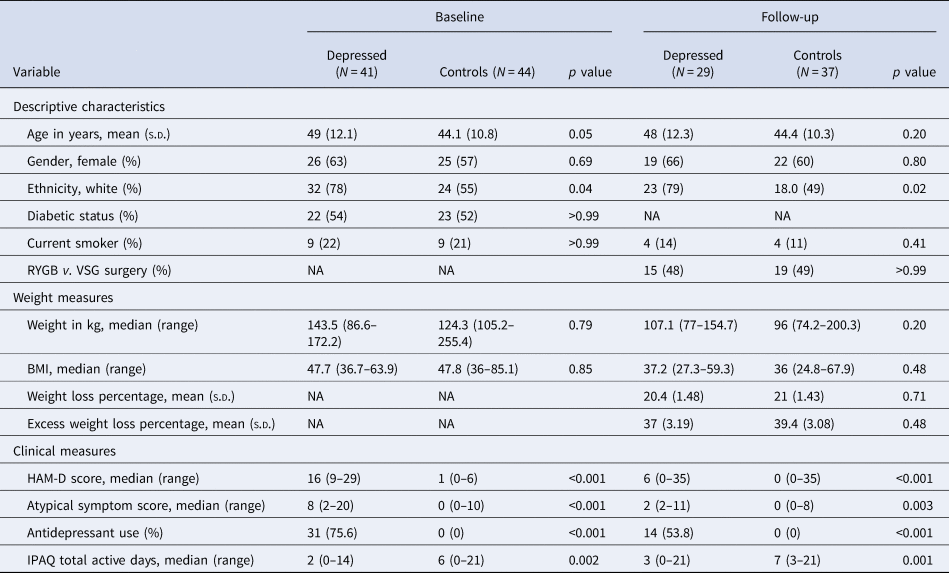
s.d., standard deviation; NA, not applicable; RYGB, Roux-en-Y gastric bypass; VSG, vertical sleeve gastrectomy surgery; BMI, body mass index; HAM-D, Hamilton Depression Rating Scale 17 for depressive symptoms; IPAQ, International Physical Activity Questionnaire.
Inflammatory markers
Before surgery, ANCOVA showed that bariatric patients with depression had significantly higher inflammation compared with controls based on their higher hsCRP levels (adjusted mean: 2.27 [CI 1.89–2.66] v. 1.52 [CI 1.08–1.95]; p = 0.02); higher IL-6 levels (adjusted mean: 0.88 [CI 0.67–1.1] v. 0.27 [CI 0.03–0.51]; p = 0.001); higher IL-6/IL-10 ratio (adjusted mean: 3.09 mg/L [CI 2.71–3.47] v. 2.22 [CI 1.79–2.64]; p = 0.006) and lower IL-4 levels (adjusted mean: 1.11 [CI 0.09–1.13] v. 0.15 [CI 0.13–1.63], p = 0.018; see Fig. 1). No significant group differences were observed for all other cytokines (see Table 2). Sensitivity analyses confirmed sample-wide correlations between higher IL-6 and IL-6/IL-10 ratio levels with more severe baseline depressive symptoms (R = 0.3 and p = 0.005; R = 0.29 and p = 0.007, respectively), while IL-4 levels were negatively correlated with baseline depressive symptoms at trend-level (R = −0.2 and p = 0.06; see online Supplementary Table S4 for all correlations).
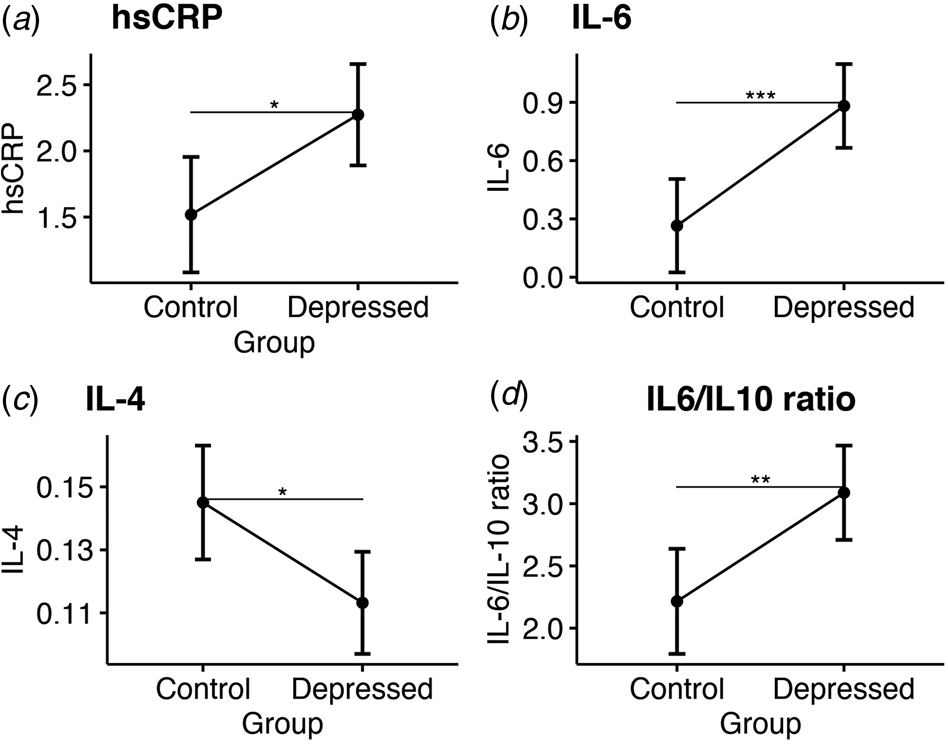
Figure 1. Group comparisons of mean serum inflammatory markers at baseline. Estimated marginal means and confidence intervals for: (a) High sensitivity C-reactive protein. (b) Interleukin 6. (c) Interleukin 4. (d) The ratio of interleukin 6 to interleukin 10.
Table 2. Serum inflammatory markers at baseline, adipose tissue inflammatory markers at time of surgery, and serum inflammatory markers at 6-month follow-up after bariatric surgery
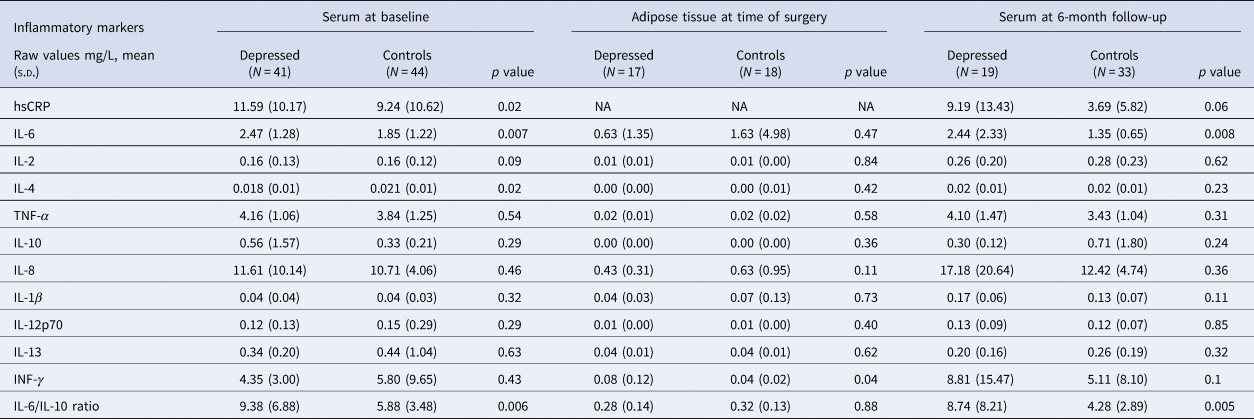
s.d., standard deviation; hsCRP, high sensitivity C-reactive protein; IL, interleukin; TNF, tumor necrosis factor; INF, interferon.
At the time of surgery, ANCOVA showed that bariatric patients with depression had significantly higher adipose INF-γ levels compared with controls (adjusted mean: −2.94 [CI −3.54 to −2.34] v. −3.97 [CI −4.7 to −3.24]; p = 0.04). All other adipose cytokines showed no significant group differences (see Table 2), and no significant correlations were observed between adipose cytokines and serum cytokines (see online Supplementary Table S5), or between adipose cytokines and depressive symptoms (see online Supplementary Table S4).
At follow-up, ANCOVA showed that bariatric patients with depression still had significantly higher IL-6 levels (adjusted mean: 0.56 [CI 0.27–0.86] v. 0.09 [CI −0.26 to 0.43], p = 0.008) and higher IL-6/IL-10 ratio (adjusted mean: 1.77 [CI 1.31–2.23] v. 0.99 [CI 0.46–1.53], p = 0.005), a trend for higher hsCRP levels (adjusted mean: 1.26 [CI 0.67 to –1.86] v. 0.6 [CI −0.1 to 1.3]; p = 0.06), but no difference in IL-4 levels (see Fig. 2). No significant group differences were observed for all other serum cytokines at follow-up (see Table 2). Sensitivity analyses confirmed sample-wide correlations between higher serum IL-6 and IL-6/IL-10 ratio levels with more severe depressive symptoms follow-up (R = 0.45 and p < 0.001; R = 0.49 and p < 0.001, respectively), but IL-4 levels were not correlated with depressive symptoms at follow-up (R = 0.13 and p = 0.4; see online Supplementary Table S4 for all correlations).
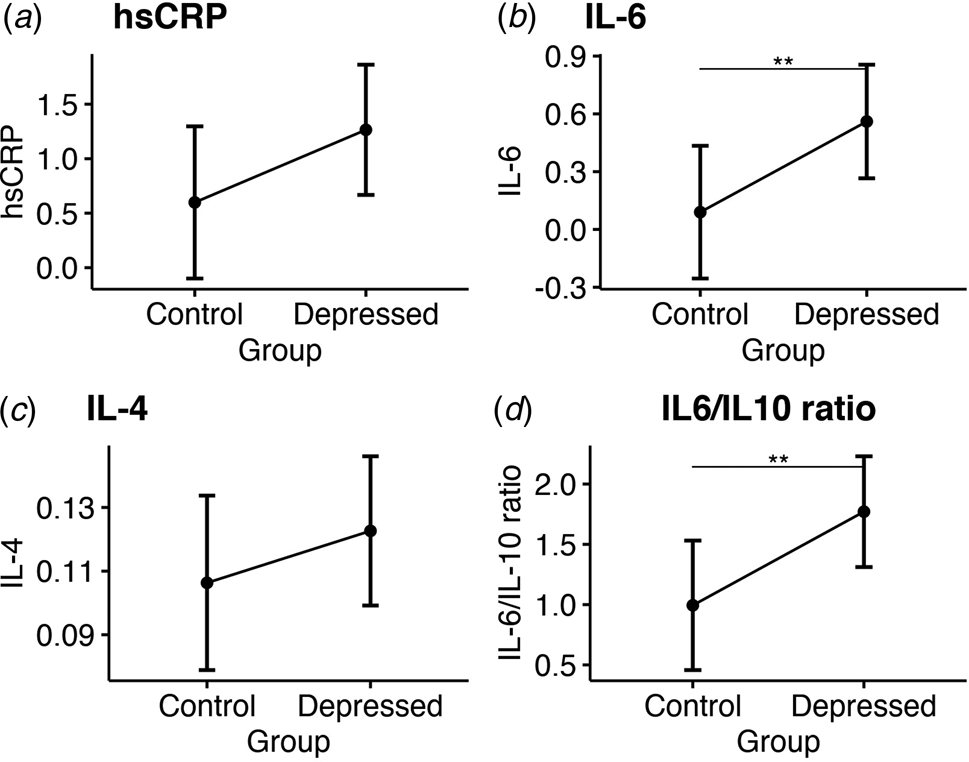
Figure 2. Group comparisons of mean serum inflammatory markers at 6-month follow-up. Estimated marginal means and confidence intervals for: (a) High sensitivity C-reactive protein. (b) Interleukin 6. (c) Interleukin 4. (d) The ratio of interleukin 6 to interleukin 10.
Predicting patient outcomes after surgery
We selected baseline inflammatory markers and clinical measures as covariates for hierarchical regression based on significant correlations with weight loss and depression severity at 6-month follow-up after surgery (see online Supplementary Table S6). Baseline predictors were adjusted against socio-demographic and clinical confounders in separate hierarchical regression models for weight loss (see online Supplementary Table S7) and depression severity (see online Supplementary Table S8).
The final multivariable regression model predicting weight loss after bariatric surgery showed that higher baseline hsCRP levels predicted reduced weight loss at 6-month follow-up (β = −0.28 [CI −0.34 to −0.04], p = 0.01), along with socio-demographic and clinical confounders including VSG surgery (as opposed to RYGB), higher age (at trend-level), female gender, diabetic status, and current smoking status (see Fig. 3a).
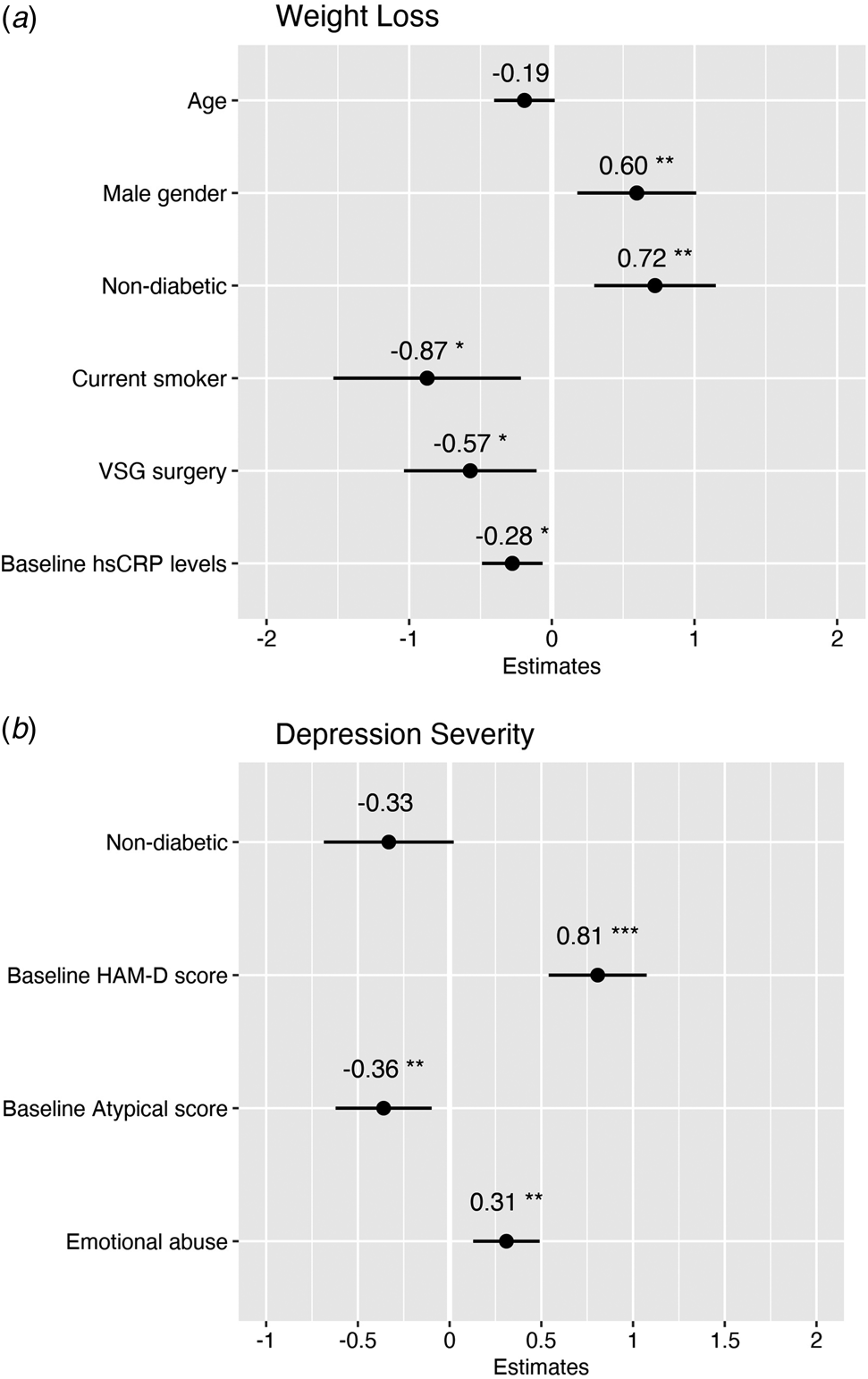
Figure 3. Multivariable regression models showing baseline predictors of weight loss and depression severity 6 months after bariatric surgery. (a) The weight loss model predicted 36% adjusted variance in weight loss at 6 months after surgery with an average prediction error rate of 5.6%. (b) The depression severity model predicted 54% adjusted variance in depressive symptoms 6 months after surgery with an average prediction error rate of 6.1%.
The final multivariable regression model predicting depression severity after bariatric surgery showed no effect of baseline hsCRP on HAM-D depressive symptoms at 6-month follow-up (β = −0.02 [CI −0.38 to 0.34], p = 0.9; see online Supplementary Table S7). Instead, higher baseline HAM-D scores and higher childhood emotional abuse scores predicted higher depression severity after surgery (β = 0.81 [CI 0.55–1.09], p < 0.001; and β = 0.31 [CI 0.22–0.85], p = 0.001, respectively). In contrast, higher baseline atypical scores predicted lower depression severity after surgery (β = −0.36 [CI −1.04 to −0.16], p = 0.008; see Fig. 3b). Of the socio-demographic or clinical variables, only non-diabetic status was associated with lower depression severity at trend-level.
Discussion
Our study is the first to show that depression diagnosis is uniquely associated with significant hsCRP, IL-6, and IL-6/10 ratio levels in patients with obesity undergoing bariatric surgery. Six months after surgery, IL-6 and IL-6/10 ratio levels were still significantly higher in patients with pre-existing depression, despite no differences in postoperative weight loss between depressed and control groups. Multivariable regression confirmed these findings, showing higher baseline inflammation predicted reduced weight loss after surgery rather than baseline depressive symptoms. In contrast with our predictions, baseline inflammation was not associated with depression outcomes after surgery. Instead, baseline depression severity and childhood emotional abuse were significant risk factors for higher depression severity after surgery. Overall, bariatric surgery led to remarkable weight loss in all patients within 6 months, and the majority of patients with pre-operative depression also experienced significant remission of their depressive symptoms, assessed by clinical interview.
Our findings of increased inflammation in the depressed group are consistent with the growing body of literature finding elevated hsCRP or IL-6 associated with depression in non-obese populations (Osimo et al., Reference Osimo, Pillinger, Rodriguez, Khandaker, Pariante and Howes2020) and our previous work showing elevated hsCRP associated with comorbid depression and overweight (McLaughlin et al., Reference McLaughlin, Nikkheslat, Hastings, Nettis, Kose, Worrell and Mondelli2022). Obesity is known to increase peripheral inflammatory markers significantly. However, our results showed that depression diagnosis was uniquely associated with increased hsCRP, IL-6, and IL-6/10 ratio and decreased IL-4 levels after controlling for BMI and socio-demographic confounders. Similar to other studies of depressed patients (Dixon, Dixon, & O'Brien, Reference Dixon, Dixon and O'Brien2003; Jha et al., Reference Jha, Cai, Minhajuddin, Fatt, Furman, Gadad and Trivedi2020; Lamers et al., Reference Lamers, Milaneschi, Smit, Schoevers, Wittenberg and Penninx2019; Moriarity, Slavich, Alloy, & Olino, Reference Moriarity, Slavich, Alloy and Olino2023; Roohi, Jaafari, & Hashemian, Reference Roohi, Jaafari and Hashemian2021), we observed significant correlations between serum inflammatory markers hsCRP, IL-6, IL-6/10 ratio, and IL-4 with higher depressive symptom severity. More recently, transcriptomic analyses of bariatric patients revealed upregulation of cytokine networks IL-6 and IL-4 associated with depression (Moisan et al., Reference Moisan, Foury, Dexpert, Cole, Beau, Forestier and Capuron2021). Given the lack of associations between adipose cytokines with depressive symptoms, it is likely that serum cytokines were responsible for driving the higher inflammation we observed in patients with depression. These convergent findings reveal a molecular signaling pathway through which inflammatory cytokine activity in the periphery increases the risk for inflammation-induced depressive symptoms via increased IL-6, IL-6/10 ratio, and decreased IL-4.
Despite the high number of studies investigating depression and weight loss in bariatric patients, our study is the first to control for the effect of inflammation on depression and weight loss outcomes. Our findings showed that high baseline inflammation could be an underlying confounder driving the association between preoperative depression and poor weight loss in bariatric patients. While recent meta-analyses suggest preoperative depression is not associated with reduced weight loss in the short to mid-term period after bariatric surgery (Dixon et al., Reference Dixon, Dixon and O'Brien2003; Dymek, Le Grange, Neven, & Alverdy, Reference Dymek, Le Grange, Neven and Alverdy2002; Fu et al., Reference Fu, Zhang, Yu, Mao and Su2021; Gill et al., Reference Gill, Kang, Lee, Rosenblat, Brietzke, Zuckerman and McIntyre2019; Kops et al., Reference Kops, Vivan, de Castro, Horvath, Costa and Friedman2020; Scholtz et al., Reference Scholtz, Bidlake, Morgan, Fiennes, El-Etar, Lacey and McCluskey2007; White et al., Reference White, Kalarchian, Levine, Masheb, Marcus and Grilo2015), other studies with extended follow-up periods found that a subset of patients with preoperative depression demonstrated reduced weight loss (da Cruz et al., Reference da Cruz, Branco-Filho, Zaparolli, Wagner, de Paula Pinto, Campos and Taconeli2018; Lai et al., Reference Lai, Aceto, Santucci, Pierro, Petrucci, Cacioppo and Raffaelli2020; Morgan, Ho, & Platell, Reference Morgan, Ho and Platell2020). Given that our results showed patients with depression were more likely to be inflamed and higher inflammation negatively influenced weight loss, it seems plausible that patients with depression would be more vulnerable to weight regain in the long term. Therefore, the poor weight loss outcomes in bariatric patients with depression could be a consequence of higher pre-operative inflammation, rather than pre-operative depression per se.
If the high inflammation in patients with depression persists and negatively affects weight loss, it may also contribute to a higher risk of severe or recurring depressive symptoms in the long term. Indeed, patients who fail to lose weight postoperatively have a higher risk for depression (Lim, Zhang, & Ho, Reference Lim, Zhang and Ho2018; van Hout et al., Reference van Hout, Verschure and van Heck2005; White, Courcoulas, & King, Reference White, Courcoulas and King2022). Although baseline inflammation was not associated with depression severity at the 6-month follow-up in our sample, the literature suggests that patients with pre-operative depression may become more vulnerable to weight regain or returning depressive symptoms 2–3 years after bariatric surgery (Gill et al., Reference Gill, Kang, Lee, Rosenblat, Brietzke, Zuckerman and McIntyre2019; Morgan et al., Reference Morgan, Ho and Platell2020; Spirou et al., Reference Spirou, Raman and Smith2020). Therefore, patients with inflammation-induced depression may be at higher risk for poor weight loss outcomes and subsequent depression relapse, but such temporal relationships may only become evident in the long term. Additionally, differences in cytokine responses may fluctuate over time in response to surgical stress and subsequent weight loss. Yet, meta-analyses have observed significant heterogeneity among changes in cytokine levels post-surgery, irrespective of the duration of follow-up or changes in BMI (Askarpour, Khani, Sheikhi, Ghaedi, & Alizadeh, Reference Askarpour, Khani, Sheikhi, Ghaedi and Alizadeh2019; Khosravi-Largani et al., Reference Khosravi-Largani, Nojomi, Aghili, Otaghvar, Tanha, Seyedi and Mottaghi2019). While our study revealed persistent heightened immune dysregulation in serum IL-6 and IL-6/10 ratio of the depressed group at 6 months post-surgery, these increases seemed unrelated to adipose tissue markers and post-operative changes in body weight, suggesting other factors, such as peripheral immune cell markers, may need to be explored further.
Finally, our results finding higher baseline depression severity and a history of childhood emotional abuse were risk factors for postoperative depression severity are consistent with other research highlighting the need for patients with severe depression and childhood trauma to receive additional psychiatric support post-surgery (King et al., Reference King, Hinerman, Kalarchian, Devlin, Marcus and Mitchell2019b; Spirou et al., Reference Spirou, Raman and Smith2020). Meta-analytic evidence has consistently identified childhood emotional abuse as a pervasive risk factor for depression risk and severity (Humphreys et al., Reference Humphreys, LeMoult, Wear, Piersiak, Lee and Gotlib2020; Mandelli, Petrelli, & Serretti, Reference Mandelli, Petrelli and Serretti2015). Moreover, in bariatric patients, higher levels of childhood emotional abuse have been shown to predict less improvement or even worsening of depressive symptoms in a dose-response manner for up to 7 years post-surgery (King et al., Reference King, Hinerman, Kalarchian, Devlin, Marcus and Mitchell2019b). As childhood emotional abuse can cause individuals to develop enduring negative cognitive beliefs, this may result in deeply internalized cognitive patterns and persistent depressive symptoms that are resistant to improvement (Jopling, Tracy, & LeMoult, Reference Jopling, Tracy and LeMoult2020), despite achieving successful weight loss through bariatric surgery. In contrast, higher baseline atypical symptoms predicted significantly lower classical depressive symptom severity after surgery. Atypical depressive symptoms are predominantly metabolic in nature (Łojko & Rybakowski, Reference Łojko and Rybakowski2017; Milaneschi et al., Reference Milaneschi, Lamers, Berk and Penninx2020), and we observed a remarkable improvement in atypical symptoms following bariatric surgery. Therefore, patients with more atypical depressive symptoms may experience a greater psychological benefit from bariatric surgery than patients with a classical presentation of depression. Identifying which subgroups of patients would benefit most from psychological treatment could improve the allocation of clinical resources in the post-surgery period (Di Lorenzo et al., Reference Di Lorenzo, Antoniou, Batterham, Busetto, Godoroja, Iossa and Silecchia2020).
Our study was limited by the relatively short 6-month follow-up period. However, the first postoperative year seems crucial in determining long-term mental health and successful weight loss (Geerts et al., Reference Geerts, Van Den Berg, Van Riel, Peen, Goudriaan and Dekker2021; Smith et al., Reference Smith, Mason, Cao, Crosby, Steffen, Garcia and Mitchell2020). Additionally, a recent clinical trial found that the optimal time to initiate psychological treatment for bariatric patients was shortly after surgery and before significant weight loss occurred (Paul et al., Reference Paul, van der Heiden, van Hoeken, Deen, Vlijm, Klaassen and Hoek2021). Therefore, data collected from early post-surgical periods may help predict which subgroups of patients are most vulnerable to experiencing poor clinical outcomes after surgery. Additionally, our study may have been limited by the significant time commitment required from participants to allow for comprehensive data collection during the study visits. Although we provided vouchers to compensate participants for their time and scheduled study visits to coincide with their hospital appointments, the financial incentive of the vouchers was minimal, which may have deterred some participants from taking part.
Our findings identifying potential risk factors for poor outcomes after bariatric surgery have clinical and research implications. Firstly, bariatric candidates with high inflammation may represent an ideal subgroup of patients for stratification in clinical trials to investigate whether anti-inflammatory medications improve postoperative weight loss or depression symptoms. Significant postoperative reductions in CRP have been associated with improved weight loss and metabolic health in bariatric patients (Illán Gómez et al., Reference Illán Gómez, Gonzálvez Ortega, Aragón Alonso, Orea Soler, Alcaraz Tafalla, Pérez Paredes and Lozano Almela2016; O'Rourke et al., Reference O'Rourke, Johnson, Purnell, Courcoulas, Dakin, Garcia and Wolfe2019), while anti-inflammatory treatments improve antidepressant response in patients with high inflammation (Nettis et al., Reference Nettis, Lombardo, Hastings, Zajkowska, Mariani, Nikkheslat and Mondelli2021; Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake and Miller2013). It remains to be seen whether bariatric patients with depression represent a distinct inflammatory immunophenotype of patients with depression or whether distinct inflammatory profiles also exist within this population (Felger & Miller, Reference Felger and Miller2020). Future studies will need to consider other peripheral blood biomarkers associated with inflammatory cytokine activity (Cavaleri et al., Reference Cavaleri, Bartoli, Capogrosso, Guzzi, Moretti, Riboldi and Carrà2023), to further clarify the immune pathways involved in depressive symptom dimensions.
In this longitudinal observational study of 85 patients with obesity undergoing bariatric surgery, depression diagnosis was associated with significantly higher serum inflammation before and 6 months after surgery. Our findings suggest that increased inflammation, rather than depression diagnosis, may be responsible for the high variability in weight loss and depression outcomes among bariatric patients. Future research should combine inflammatory biomarkers and clinical data in longitudinal prediction models to accurately identify risk factors for poor outcomes after bariatric surgery. Understanding the inflammatory pathways underlying the vicious cycle between depression and obesity, and how interventions such as bariatric surgery may break this cycle, may inform future strategies addressing the rising prevalence of these conditions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723002283.
Acknowledgements and Disclosures
The study received funding support from the National Institute for Health and Care Research (NIHR) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust, and King's College London. Professor Mondelli is also funded by MQ: Transforming Mental Health (Grant: MQBF/1 and MQBF/4) and the Medical Research Foundation (Grant: MRF-160-0005-ELP-MONDE). Ms Milton received funding through the Neuro-Immune Interactions in Health & Disease Wellcome Trust PhD Training Programme (218452/Z/19/Z) at King's College London. Ms Patsalos received salary support from the National Institute of Health and Care Research (NIHR) Maudsley Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust (SLaM) and King's College London (KCL). We thank the staff at King's College Hospital, the study participants, and their families for their contribution to this research. Part of this work was presented as a poster at the 2021 British Association of Psychopharmacology conference in London, UK. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. Professor Mondelli received research funding from Johnson & Johnson as part of a research program on depression and inflammation, but this is unrelated to the work presented in this study. No other disclosures were reported.










