Introduction
The chelicerate order Xiphosurida, generally known by the colloquial misnomer ‘horseshoe crabs,’ is among one of the rarest of invertebrate taxa, mostly owing to their unmineralized exoskeleton and predilection for marginal environments that are so rare in the stratigraphic record (Babcock et al., Reference Babcock, Merriam and West2000; Loveland and Botton, Reference Loveland and Botton2015; Lamsdell, Reference Lamsdell2016). Thus, the discovery of Middle Triassic horseshoe crab material adds significantly to our understanding of the group. Xiphosurida have existed for ~480 Myr (Lamsdell, Reference Lamsdell2013, Reference Lamsdell2016), with the earliest unequivocal representatives found in the Upper Ordovician of Manitoba, Canada (Rudkin et al., Reference Rudkin, Young and Nowlan2008), apparently preceded by finds of putative Xiphosurida from the Lower Ordovician of Morocco (Van Roy et al., Reference Van Roy, Orr, Botting, Muir, Vinther, Lefebvre, el Hariri and Briggs2010). Only four species of horseshoe crabs exist today, all of which are members of Limulidae Zittel, Reference Zittel1885 (=Mesolimulidae Størmer, Reference Størmer1952) and characterized by their large crescentic prosomal shield and the fusion of the opisthosomal tergites (Lamsdell and McKenzie, Reference Lamsdell and McKenzie2015). The recently published study of the Xiphosura (equivalent to total group Xiphosurida) phylogeny (Lamsdell, Reference Lamsdell2016) has interesting implications. First of all, they suggested that horseshoe crabs have independently invaded the non-marine realm at least five times during their long evolution. Secondly, horseshoe crabs have had rather a complex evolutionary history not only in the Paleozoic, but also in the Mesozoic.
The aim of this paper is to describe a new species of horseshoe crab, Limulitella tejraensis n. sp., from the Middle Triassic of southern Tunisia (Saharan Platform). Limulitella represents the most basal representative of the family Limulidae (Lamsdell, Reference Lamsdell2016), which is a clade of Triassic to Recent horseshoe crabs that exhibits no vestige of segmentation dorsally in the opisthosoma (Riek and Gill, Reference Riek and Gill1971), and the axis of the thoracetron bears a dorsal keel (Lamsdell, Reference Lamsdell2016).
Geologic setting
Triassic deposits of southern Tunisia crop out widely in the Jeffara domain (Fig. 1.1, 1.2). The main outcrops are exposed along a NW-SE trending belt from Jebel Tebaga of Medenine, which includes the Tejra outcrops, to the Libyan border. The Triassic succession is very thick (>2000 m), especially in the Jeffara Basin, and is dominated in its lower part by red-beds, which usually lie unconformably on upper Permian rocks (Busson, Reference Busson1967; Bouaziz, Reference Bouaziz1986; Kilani-Mazraoui et al., Reference Kilani-Mazraoui, Razgallah-Gargouri and Mannai-Tayech1990; Kamoun et al., Reference Kamoun, Peybernès, Martini, Zaninetti, Vila, Trigui and Rigane1998, Reference Kamoun, Peybernès and Fauré1999, Reference Kamoun, Peybernès, Ciszak and Calzada2001; Dridi and Maazaoui, Reference Dridi and Maazaoui2003). The Tunisian Triassic paleogeographic evolution results from the disassembly of Pangaea in the early Mesozoic, when the North African Platform became a broad Tethyan-facing passive continental margin (Soussi et al., Reference Soussi, Abbes and Belayouni1998, Reference Soussi, Cirilli and Abbes2001; Kamoun et al., Reference Kamoun, Peybernès, Ciszak and Calzada2001; Bouaziz et al., Reference Bouaziz, Barrier, Soussi, Turki and Zouari2002; Courel et al., Reference Courel, Ait Salem, Benaouiss, Et-Touhami, Fekirine, Oujidi, Soussi and Tourani2003).
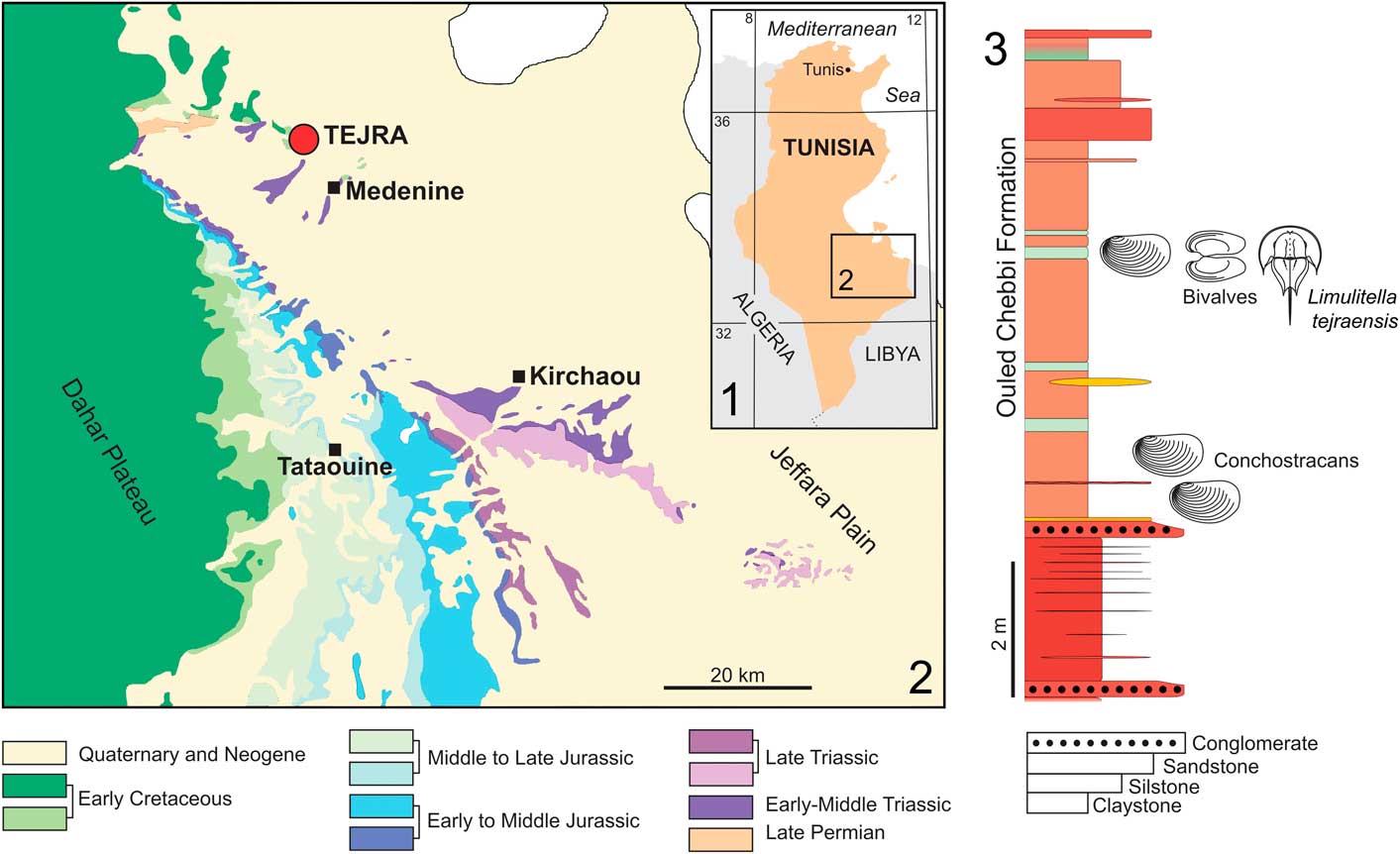
Figure 1 Location of the studied area in Tunisia: (1, 2) geological map of the Jeffara Plain and Dahar Plateau (Medenine/Kirchaou/Tataouine area) with position of the Tejra site; (3) columnar section of the Ouled Chebi Formation exposed at the lower part of the Tejra 2 clay-pit, showing the horizon with occurrence of the studied Limulitella material.
The material studied herein comes from a locality called either Tajera Skhira (e.g., Biely and Rakus, Reference Biely and Rakus1991) or Tejra Sghira (e.g., Baccour et al., Reference Baccour, Medhioub, Jamoussi, Mhiri and Daoud2008). The Triassic succession there is exposed in a monoclinal structure with beds dipping southwest. It is unconformably overlain by middle Callovian, shallow-marine Jurassic carbonates. Bed-by-bed logging of the sites around Tejra resulted in construction of a detailed stratigraphic section that is ~100 m thick. However, only part of this section (the main Tejra outcrop) was analyzed in detail in the field and studied paleontologically (Soussi et al., Reference Soussi, Niedźwiedzki, Tałanda, Dróżdż, Sulej, Boukhalfa, Mermer and Błażejowski2016).
The Tejra section can be subdivided into four distinctive units. The first unit represents the basal part of the section and is ~25 m thick. It is composed of red claystones comprising gray to green, thin claystones interbedded with numerous medium- to coarse-grained and well-sorted sandstones (Fig. 1.3). Local desiccation features observed are numerous ripple marks on the upper surfaces of thin sandstone layers. The brown and green claystone horizons encountered at the base of this unit are particularly rich in conchostracans. A succession of two gray-yellow beds bounding a brown bed yielded an abundant and a very diverse freshwater fauna (Fig. 2.1). This is situated ~5 m above a basal conglomerate, composed of claystones irregularly interbedded with well-sorted and rounded grains of quartz (~1–2 mm in diameter). Most of the fossils are derived from yellowish sediments at the top of the lower gray/green bed (Fig. 2.2). The fauna is represented by conchostracans (Fig. 2.3), horseshoe crabs, freshwater bivalves (Fig. 2.4, 2.5), rare microconchids, and lingulides. Desiccation cracks, small burrows, and casts from plant roots are also present in this horizon.

Figure 2 Details of the fossil-bearing strata, lower part of the Tejra section: (1) exploration of fossil-bearing deposits represented by red claystone and siltstone intercalated with thick greenish or yellowish claystone beds from the lower part of the section (arrow indicates freshwater horizon with Limulitella remains); (2) details of the greenish claystone with freshwater fauna; (3) conchostracan from freshwater horizon, large form (species B) described as cf. Dictyonatella sp. (4, 5) Bivalves from the freshwater horizon: (4) Unionites cf. brevis; (5) Unionites cf. longus. Scale bar in (3–5) is 10 mm. Hammer for scale in (2) represents 30 cm.
The second unit is ~30 m thick. It is clay dominated, but contains numerous sandstone interbeds. At the base, two thick layers of medium-grained red sandstone, interbedded with a 2 m thick red claystone yield a rich fauna of marine or brackish bivalves, microconchids, gastropods, and lingulides.
The third unit is ~35 m thick. It is composed mainly of reddish-brown siltstones and claystones and comprises a sandstone bed marker in its middle part showing numerous invertebrate ichnofossils in its lower part. The fauna includes conchostracans, lingulides, and rare partially preserved horseshoe crabs, although the latter were found only at the base of the unit.
The fourth unit is ~10 m thick. It mainly comprises brown claystone with thin green beds and contains a fauna of conchostracans and lingulids, rare gastropods, rare horseshoe crabs, and microconchids. The claystones represent deposition in floodplains and lakes and the associated sandstone and siltstone interbeds are interpreted as deposits resulting from intermittent flood events.
Materials and methods
The described material comes from two abandoned clay pits in Tejra area near Medenine (Fig. 1). The samples collected in 2013 and 2014 from the Tejra section yielded a total of 21 specimens, including one almost completely articulated exoskeleton (Fig. 3.1). The other twenty specimens are incomplete, being preserved as isolated fragments of the prosoma and opisthosoma (Fig. 3.3–3.8). The horseshoe crab-bearing rocks are green, rather poorly lithified claystones and siltstones.

Figure 3 (1) Articulated exoskeleton of the holotype (ZPAL V.46/101p); (2) diagram of morphological features exhibited on the dorsal carapace of Limulitella tejraensis n. sp.; not observed on the holotype are the opisthosomal (moveable) spines; (3) incomplete exoskeleton (ZPAL X.46/106); (4) complete opisthosoma (ZPAL X.46/109); (5) complete opisthosoma (ZPAL X.46/102); (6) incomplete exoskeleton (ZPAL X.46/120); (7, 8) the part (ZPAL X.46/103p) and counterpart (ZPAL X.46/103n) of the flattened, complete prosoma (ZPAL X.46/103p).
All available biostratigraphic and sedimentologic data comprising the latest developments in the Middle to Upper Triassic rocks of Algeria, Tunisia, and Libya were used for construction of a regional stratigraphic cross section and elaboration of an updated stratigraphic framework (Soussi et al., Reference Soussi, Niedźwiedzki, Tałanda, Dróżdż, Sulej, Boukhalfa, Mermer and Błażejowski2016). Specimens were coated with ammonium chloride and photographed using a Canon EOS 400D digital camera.
Repository and institutional abbreviation
The collected material is housed at the Institute of Paleobiology, Polish Academy of Science in Warsaw (collection ZPAL V.46).
Systematic paleontology
Phylum Arthropoda Latreille, Reference Latreille1829
Order Xiphosurida Latreille, Reference Latreille1802
Family Limulidae Zittel, Reference Zittel1885 (=Mesolimulidae Størmer, Reference Størmer1952)
Genus Limulitella Størmer, Reference Størmer1952
Type species
Limulites bronnii Schimper, Reference Schimper1853.
Diagnosis
“Mesolimulidae with postmedian margin of genal angle forming an angle with anterolateral margin of narrow subtriangular abdomen, a median keel may occur on axis” (Størmer, Reference Størmer1952, p. 637).
Remarks
Placement of Limulitella within Limulidae is based upon affinities of that genus with Mesolimulus Størmer, Reference Størmer1952, who allied the two in the Mesolimulidae. Mesolimulidae has subsequently been synonymized with Limulidae (Riek and Gill, Reference Riek and Gill1971). Limulitella has been interpreted by some as being more closely associated with Paleozoic genera embraced within Paleolimulidae Raymond, Reference Raymond1944 (Riek and Gill, Reference Riek and Gill1971; Holland et al., Reference Holland, Erickson and O’Brien1975; Feldmann et al., Reference Feldmann, Schweitzer, Dattilo and Farlow2011). Although the family placement is not the focus of this work, it is noteworthy that general absence of visible segmentation on the axial part of the opisthosoma argues in favor of placement within the Limulidae. Limulitella Størmer, Reference Størmer1952 (= Limulites Schimper, Reference Schimper1853) includes: Limulitella cf. liasokeuperinus (Braun, Reference Braun1860), brackish-marine, Pliensbachian (Early Jurassic) (this particular specimen was lost during World War II); L. vicensis (Bleicher, Reference Bleicher1897), marine, Early Triassic; L. henkeli Fritsch, Reference Fritsch1906, marine, Early Triassic; L. volgensis Ponomarenko, Reference Ponomarenko1985, brackish-marine, Early Triassic; L. bronnii (Schimper, Reference Schimper1853), Middle Triassic; and three unnamed specimens referred to Limulitella by Hauschke et al. (Reference Hauschke, Wilde and Brauckmann2004, Madagascar), Hauschke and Wilde (Reference Hauschke and Wilde2008, Germany), and Hauschke et al. (Reference Hauschke, Oosterink and Wilde2009, the Netherlands).
Limulitella tejraensis new species
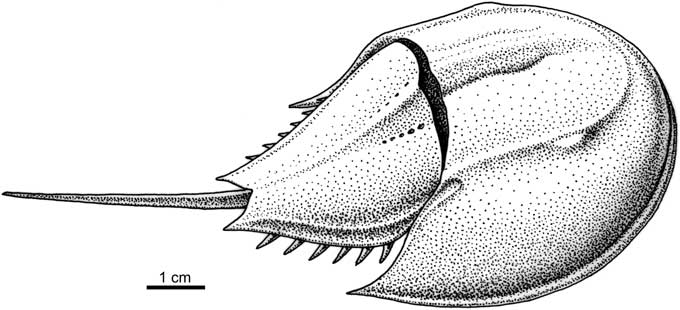
Figure 4 Reconstruction of Limulitella tejraensis n. sp. The movable spines are not present on the fossils.
Holotype
ZPAL V.46/101p (Fig. 3.1).
Type locality and horizon
Tejra, Ouled Chebbi Formation (Anisian–early Ladinian, Middle Triassic), Medenine area, Sahara Platform, southern Tunisia.
Geographic range
Known from the type locality only.
Diagnosis
Prosoma with depressed occipital bands, broad at the ophthalmic ridge becoming thinner and disappearing at the genal spines. Ophthalmic ridge extends in S-shaped line from ocelli along the carapace inner edge as far as the genal angle. Posterior margin of opisthosoma lacks small spines.
Description
Prosoma wider than long (average length 16.7 mm; average width 31.1 mm), weakly to moderately vaulted. Prosomal rim uniform, narrow (Fig. 3.1, 3.3). Anterior margin semicircular, with lateral margins parallel to median axis of carapace, ending posteriorly in long acute genal spines (Fig. 3.7, 3.8). Cardiac lobe smooth, narrowing anteriorly with sinuous margin and obscure nodose anterior, extends ~50% of prosoma length, terminating anteriorly at slightly visible ocelli (Fig. 3.1, 3.3, 3.6, 3.7). Large lateral (compound) eyes posterior to midlength on well-defined ophthalmic ridges (Fig. 3.6, 3.7). Ophthalmic ridge forming rounded structure, inscribing an S-shape within an inner ridge of prosoma to genal spines (Fig. 3.2, 3.7). The genal spines curve inwardly and posteriorly along outer ridge of prosoma. Depressed occipital bands are broad at the ophthalmic ridge, becoming thinner and then disappearing at the genal spines (Fig. 3.1).
Unsegmented opisthosoma small (average length 12.2 mm; average width, 19.1 mm), triangular, weakly to moderately vaulted in cross section (Fig. 3.4, 3.5). Axial ridge well defined (Fig. 3.5) in the anterior part, bounded by five pairs of depressed entapophoseal pits (apodemes), decreasing in size posteriorly along diagonal axial furrows. Longitudinal ridges form inner margin of opisthosomal flanks, which are separated from central part of opisthosoma by distinct pleurae. Not observed are the opisthosomal (moveable) spines. Flanks extend along S-shaped pleurae. Posterior margin relatively deep and triangular, terminating in two distinct large marginal spines. Telson triangular in cross section. Venter not preserved in specimens.
Etymology
After the type locality.
Other material
Five paratypes, ZPAL X.46/102; ZPAL X.46/103p,n; ZPAL X.46/106; ZPAL X.46/109; ZPAL X.46/120. Others specimens: ZPAL X.46/104; ZPAL X.46/105; ZPAL X.46/107; ZPAL X.46/108; and ZPAL X.46/110-119.
Discussion
Comparison of Limulitella tejraensis n. sp. with other Triassic limulines
Triassic horseshoe crabs are considered to have a relatively poor fossil record, with only nine genera having been described: Limulitella Størmer, Reference Størmer1952, known from Germany (Fritsch, Reference Fritsch1906; Hauschke and Wilde, Reference Hauschke and Wilde2008), France (Schimper, Reference Schimper1853; Bleicher, Reference Bleicher1897), the Netherlands (Hauschke et al., Reference Hauschke, Oosterink and Wilde2009), Madagascar (Hauschke et al., Reference Hauschke, Wilde and Brauckmann2004), and Russia (Ponomarenko, Reference Ponomarenko1985); Mesolimulus Størmer, Reference Størmer1952 from Spain (Vía, Reference Vía1987); Tachypleus Leach, Reference Leach1819 (=Heterolimulus Vía Boada and De Villalta, Reference Vía Boada and De Villalta1966) from France; Tarracolimulus Romero and Boada, Reference Romero and Boada1977 from Spain; Psammolimulus Lange, Reference Lange1923 from Germany (Lange, Reference Lange1923; Meischner, Reference Meischner1962); Yunnanolimulus Zhang et al., Reference Zhang, Hu, Zhou, Lü and Bai2009 from south-west China (Hu et al., Reference Hu, Zhang, Chen, Zhou, Lü, Xie, Wen, Huang and Benton2011); Paleolimulus Dunbar, Reference Dunbar1923 from Germany (Hauschke and Wilde, Reference Hauschke and Wilde1987, Reference Hauschke and Wilde2000); and two genera described from freshwater strata of Middle Triassic age of New South Wales (Riek, Reference Riek1968; Pickett, Reference Pickett1984): Australolimulus Riek, Reference Riek1955 and Dubbolimulus Pickett, Reference Pickett1984. Dubbolimulus has been previously considered to be a synonym of Paleolimulus (Lamsdell, Reference Lamsdell2016). With respect to the opisthosoma, which is conspicuously smaller than the carapace, the three last genera are quite different from forms described in this paper, and some of them exhibit extremely aberrant morphologies. The opisthosomal (moveable) spines are not present on the described specimens. However, on the reconstruction of Limulitella tejraensis n. sp. we have drawn moveable spines, taking into account the morphologically closest representative of the genus, Limulitella bronnii (Schimper, Reference Schimper1853) from France, illustrated by Gall and Grauvogel-Stamm (Reference Gall and Grauvogel-Stamm1999).
The morphological phylogenetic analyses recently carried out by Lamsdell (Reference Lamsdell2016) demonstrated that Limulitella is a polyphyletic genus. Of all known Triassic horseshoe crabs, Limulitella tejraensis n. sp. seems to be almost identical to the Limulitella forms from France, Germany, and Madagascar. Certainly, the morphological features of L. tejraensis are remarkably similar to those of L. bronnii (Schimper, Reference Schimper1853) from France, illustrated by Gall and Grauvogel-Stamm (Reference Gall and Grauvogel-Stamm1999; see also Röhling and Heunisch, Reference Röhling and Heunisch2010). The shape of the ophthalmic ridge, S-shaped inscribing within inner ridge of the prosoma to the characteristic genal angles are the main features that distinguish L. tejraensis n. sp. from L. bronnii. In both forms, the unsegmented opisthosoma is small with flanks extending along S-shaped pleurae and the posterior margin is significantly cleft triangularly in the posterior part, which in L. bronnii bears two shorter distinct marginal spines. The posterior margin of L. tejraensis n. sp. lacks those spines.
Limulitella bronnii was described and sketched by Schimper (Reference Schimper1853) from the early Middle Triassic (Anisian) ‘Grès à Voltzia’ Formation of eastern France, which represents an environment interpreted as a refugium for terrestrial communities during the end-Permian mass extinction and its Triassic aftermath (Gall and Grauvogel-Stamm, Reference Gall and Grauvogel-Stamm2005). In the detailed investigations of Lamsdell (Reference Lamsdell2016), L. bronnii is placed within the Limulidae, next to genera forming the xiphosurid crown group (the extinct Crenatolimulus [Feldmann et al., Reference Feldmann, Schweitzer, Dattilo and Farlow2011] and the extant Carcinoscorpius, Limulus, and Tachypleus).
Preservation and habits of Limulitella tejraensis n. sp
The state of preservation of the material found at Tejra is not ideal, owing to the nature of its compaction, as indicated by wrinkling along the anterior of the prosomae. The vast majority of the specimens are incomplete (i.e., preserved as isolated prosomal and opisthosomal fragments) and represent all four stages of disarticulation (Fig. 3), as observed by Babcock et al. (Reference Babcock, Merriam and West2000). The specimens described here are relatively small, and with respect to their presumed close relationship with extant limulines they mostly probably represent the remains of juveniles only. Jurassic horseshoe crabs known from the fossil record seem to represent juvenile forms (Błażejowski, Reference Błażejowski2015; Błażejowski et al., Reference Błażejowski, Gieszcz, Brett and Binkowski2015, Reference Błażejowski, Gieszcz and Tyborowski2016). The giant limulid trackways, Kouphichnium lithographicum Oppel, Reference Oppel1862, reported from Germany and France (Schweigert, Reference Schweigert1998; Gaillard, Reference Gaillard2011) seem to support this interpretation.
Within the strata containing Limulitella tejraensis n. sp., thin-shelled bivalves and clam shrimp (conchostracans) are present (Fig. 3.8), and these may have provided a diet for the horseshoe crabs. The diet of fossil and the modern Atlantic Limulus is highly diverse and consists of a variety of small marine organisms, including soft-shelled bivalves, gastropods, polychaetes, and crustaceans (Botton, Reference Botton1984; Botton and Ropes, Reference Botton and Ropes1989; Kin and Błażejowski, Reference Kin and Błażejowski2014).
Sedimentological and paleontological data from the section that yielded the Limulitella tejraensis n. sp. specimens suggest brackish/freshwater conditions. Forms inhabiting such environments are known from the fossil record since the late Paleozoic (Lamsdell, Reference Lamsdell2016). In his phylogenetic analysis, Lamsdell (Reference Lamsdell2016) demonstrated that limulines colonized non-marine environments many times throughout their evolutionary history. In addition to the suborder Limulina, the Belinurina are a common component of late Carboniferous to early Permian freshwater/brackish coal swamps (Filipiak and Krawczyński, Reference Filipiak and Krawczyński1996; Anderson, Reference Anderson1997; Anderson and Selden, Reference Anderson and Selden1997; Lamsdell, Reference Lamsdell2016). The loss of marine habitat and colonizing of brackish/freshwater environments are probably direct effects of the turbulent history of shallow-marine ecosystems at the end of Paleozoic (Foster and Twitchett, Reference Foster and Twitchett2014). Rapidly alternating cool and warm periods during the ensuing Carboniferous Ice Age caused deep changes in the inhabitable environments (DiMichele et al., Reference DiMichele, Pfefferkorn and Gastaldo2001; Scheffler et al., Reference Scheffler, Hoernes and Schwark2003; Peyser and Poulsen, Reference Peyser and Poulsen2008). Coastlines fluctuated widely owing to local basin subsidence and worldwide sea-level changes (Haq and Shutter, Reference Haq and Shutter2008). Continents aggregated, forming Pangea; coastline length decreased; more common deltaic environments supported fewer corals, crinoids, and bryozoans (Stanley and Powell, Reference Stanley and Powell2003; Veron, Reference Veron2008), and many groups of animals, including bivalves, gastropods, bony fish, and horseshoe crabs, were forced to adapt to freshwater/brackish environments (Wesselingh, Reference Wesselingh2007; Sallan and Galimberti, Reference Sallan and Galimberti2015; Lamsdell and Selden, Reference Lamsdell and Selden2016).
Environmental conditions changed in the late Permian, when, as a result of decreasing glaciations, the interiors of continents became drier and brackish/freshwater areas were probably reduced (Erwin, Reference Erwin1993; Twitchett et al., Reference Twitchett, Looy, Morante, Visscher and Wignall2001; Clarkson et al., Reference Clarkson, Kasemann, Wood, Lenton, Daines, Richoz, Ohnemueller, Meixner, Poulton and Tipper2015). At that time, the largest mass extinction (end-Permian extinction) recorded in the history of life on Earth began (Shen et al., Reference Shen, Cao, Henderson, Wang, Shi, Wang and Wang2006). Although it affected many groups of organisms in many different ecosystems, shallow-marine communities suffered preferentially. The current fossil record indicates that the belinurines did not survive the late Paleozoic environmental changes. A few horseshoe crab taxa, including ancestors of the Triassic genus Limulitella, were presumably so tolerant of changing conditions throughout the late Paleozoic that they survived the end-Permian mass extinction. In the early Mesozoic, the ancestral survivors presumably evolved into the Limulidae horseshoe crab lineage, which survives into modern times. In the Early–Middle Triassic, some lineages with unusual or aberrant morphologies also evolved (e.g., Austrolimulus). The late Early to early Middle Triassic represents a time of recovery following the end-Permian mass extinction (Erwin, Reference Erwin2006; Knoll et al., Reference Knoll, Bambach, Payne, Pruss and Fischer2007; Hu et al., Reference Hu, Zhang, Chen, Zhou, Lü, Xie, Wen, Huang and Benton2011; Chen and Benton, Reference Chen and Benton2012). Thus, it can be considered an important stage in the evolution history of Limulina. Despite the poor state of preservation, horseshoe crabs known from the Lower Triassic (Olenekian) from Ankitokazo Basin, Madagascar (Hauschke et al., Reference Hauschke, Wilde and Brauckmann2004) and Vetluga Series of Russia (Ponomarenko, Reference Ponomarenko1985) were identified as Limulitella. Worldwide distribution of this group in various localities and different ecosystems provides evidence for rapid adaptive radiation of the group shortly after the biotic crisis, developing adaptive capability for survival in divergent environmental niches. The assumed high degree of environmental tolerance of the genus Limulitella reflects their ability to exist in both marine habitats and freshwater/brackish environments when forced to do so. All three extant genera exhibit a high tolerance to changes of salinity in inhabited areas (Shuster, Reference Shuster1982; Ehlinger and Tankersley, Reference Ehlinger and Tankersley2009). The Indian species, Carcinoscorpius rotundicauda, is observed to migrate astonishing distances from the mouth up to 150 km to the source area of the Hooghly (Ganges) River (Annandale, Reference Annandale1922).
Thus, the evolutionary success of horseshoe crabs results in its most important part from the specialization, which played a key role during the re-establishment of the biota after the end-Permian extinction event.
Conclusion
Because xiphosurid arthropods are extremely rare in the fossil record, the recent discovery of the Middle Triassic fossils are of considerable importance in bridging the existing gap in our knowledge of limulid distribution and diversification during the early Mesozoic. The state of preservation and associated fauna indicate that the depositional area may have been a feeding zone for Limulitella tejraensis n. sp. While this new discovery of Triassic horseshoe crabs has only a limited bearing on phylogenetical relationships within the group, it certainly sheds new light on several aspects of the origin of extant limulines and the presumed role of specialization during the end of Paleozoic crisis.
Acknowledgments
We would like to thank, several institutions that have funded our research work in Tunisia. The research in 2013 was supported by the Polish Ministry of Science and Higher Education (grant number 7986/B/P01/2011/40, grant of T. Sulej and G. Niedźwiedzki). We thank R.M. Feldmann (Kent State University) and P. Gieszcz (Independent Center for Advanced Research machinic.it) for many useful suggestions and correction of English. The field study in 2014 was financed by the University of Warsaw through the Advisory Board for Student Scientific Movement (Project: 51/I/2014), the University of Warsaw Students’ Union (Project: II/539/1120/2014), the Faculty of Biology of University of Warsaw, the Department of Paleobiology and Evolution of University of Warsaw, and the Foundation of University of Warsaw. G. Niedźwiedzki is currently funded by a grant awarded to P.E. Ahlberg (Uppsala University). We would like to sincerely thank P. Walsh (University of Silesia) for many useful suggestions and linguistic correction. We are also very grateful to M. Tałanda, D. Dróżdż, J. Mermer, K. Biernacki, J. Tałanda, M. Pindakiewicz, and A. Tobolska for their help during fieldwork in 2014. Finally, we are grateful to the UR11 ES 15 from the University of Tunisi El Manar for facilitating the two field missions organized in southern Tunisia in 2014.








