A nationwide outbreak of carbapenem-resistant Enterobacteriaceae (CRE) emerged in Israel in 2006, with nosocomial spread of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. A national intervention was implemented in all acute-care hospitals including active surveillance of high-risk patients and carrier isolation and cohorting. With these efforts, the outbreak was partially contained,Reference Schwaber and Carmeli 1 yet carbapenemase-producing Enterobacteriaceae (CPE) remain still widespread in most of the large medical centers in Israel. Moreover, new types of CPE have emerged, including NDM-1– and OXA-48–producing Enterobacteriaceae.
OXA-48 enzymes, of the class D carbapenemases, were initially identified in TurkeyReference Poirel, Heritier, Tolun and Nordmann 2 in K. pneumoniae and have recently emerged as a predominant carbapenemase type in various Enterobacteriaceae in other countries.Reference Mataseje, Boyd and Fuller 3 The first cases of OXA-48–producing CPE in Israel were described in patients coming from the Palestinian AuthorityReference Adler, Solter and Masarwa 4 , Reference Goren, Chmelnitsky, Carmeli and Navon-Venezia 5 and from Syrian wounded refugees treated in Israeli hospitals.Reference Lerner, Solter and Rachi 6
Carbapenemase-producing Enterobacteriaceae can produce a broad spectrum of infections; they associated with high mortality and very limited therapeutic options. Particularly troubling are Serratia and Proteus, which are innately resistant to colistin, one of the last-resort therapeutic options. Patient-to-patient cross transmission is the main mechanism of spread of multidrug-resistant (MDR) Enterobacteriaceae nosocomial outbreaks. Colonized patients are considered the most important reservoir, and infection control breaks in hand hygiene via healthcare workers is considered the predominant transmission chain.Reference Friedman, Carmeli, Walton and Schwaber 7 Environmental reservoirs have not been frequently reported as the source for such outbreaks.
Herein, we describe a prolonged ICU S. marcescens outbreak, at an ICU of a large tertiary-care medical center, which was successfully contained by a combined intervention after recognizing the sink traps as the source of transmission.
Methods
Setting and CPE surveillance and isolation policy
The Sheba Medical Center (SMC) is a tertiary-care academic medical center in central Israel, affiliated with the Tel Aviv University; it has 1,600 beds for both acute-care and rehabilitation patients, with 96,800 annual admissions to the acute-care hospital. The general ICU consists of 16 active beds for medical and surgical patients, with ~470 admissions per year. The mean ICU hospitalization duration is 9.6 days. The patient-to-nurse ratio is 2:1. Each bed is in a semi-closed space separated by glass walls on 2 sides. The water supply and the wastewater removal system in the ICU consists of 22 sinks, including a sink in each patient unit at the entrance to the room, 4 additional sinks in the general space, and 2 sinks in the staff toilet and dressing rooms.
Active surveillance CPE screening of all ICU patients has been in place since 2007 and includes admission as well as twice-weekly rectal screening. All high-risk patients are placed in pre-emptive isolation pending admission surveillance culture results. Once a patient is detected as a CPE carrier, he is moved to a designated cohort space with dedicated nursing staff and equipment.
Case definition and patient data
A case was defined as an ICU hospitalized patient with OXA-48–producing Enterobacteriaceae isolated either from surveillance cultures or from clinical cultures obtained at the SMC since 2016. Data collected from the electronic medical files included date of admission, date of CPE acquisition, source of admission (home, nursing home, etc), admission diagnosis, comorbidities, length of stay (LOS) until and after CPE acquisition, type of clinical infection, and outcome.
Microbiological methods
Clinical isolates were identified following Clinical and Laboratory Standards Institute (CLSI) guidelines. Antibiotic susceptibility was determined using the disk diffusion method. Minimum inhibitory concentrations (MICs) of carbapenems were determined using the Etest (AB Biodisk). The MIC break points were defined according to CLSI guidelines. Surveillance rectal samples and environmental samples were obtained using Copan Amies sterile transport swabs (Copan Diagnostics, Murrieta, CA). The rectal swab samples were streaked onto Chromagar KPC plates (Hy Laboratories, Rehovot, Israel) and incubated overnight at 35°C in ambient air. Suspicious colonies were identified using matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS). Carbapenemase genes were identified by polymerase chain reaction (PCR) using the Xpert Carba-R cartridge (GeneXpert, Cepheid, Sunnyvale, CA).
Molecular characterization of the outbreak and environmental isolates was performed using pulsed-field gel electrophoresis (PFGE),Reference Ribot, Fair and Gautom 8 where XbaI-digested Serratia DNA embedded in agarose plugs were subjected to PFGE analysis at 14°C using CHEF-DR III system (Bio-Rad, Hercules, CA).
Outbreak investigation
During October 2016, after 6 patients with OXA-48–producing S. marcescens were detected, enhanced control measures were undertaken, including increased hand hygiene observations as well as educational sessions. Thorough cleaning of all surfaces and medical devices with 1,000 PPM sodium hypochlorite and quaternary ammonium, accordingly, was carried out. The number of ICU patients was cut from 16 to 8. The outbreak was partially contained, but a few weeks later, during March 2017, new acquisitions of OXA-48–producing S. marcescens were again identified.
At this point, we took a new investigational approach by engaging the ICU team in the process. We conducted 2 workshops (8 hours each) for the whole ICU team, including physicians, nurses, cleaning team, pharmacist, and social worker. The workshop included discussion groups that investigated potential infection control breaks that could explain the outbreak using a root-cause analysis with the fishbone visual approach (http://www.siliconfareast.com/ishikawa.htm).
Environmental sampling
Initial environmental sampling included swabs from beds, ventilators, mattresses, care carts, high-touch surfaces (eg, monitor and computer keyboards), IVAC pumps, several solutions, faucets, and water samples. Following the root-cause analysis, the water environment was suspected to be the source of transmission, and further environmental sampling included water samples, faucets, water pipes, sink surfaces, sink outlets, sink traps, and drain systems.
Results
Since 2007, when routine screening for CRE began in our hospital, and until 2016, 294 ICU patients acquired CRE, of whom ~70% had Klebsiella pneumoniae and only a single case had Serratia marcescens. Routine determination of the carbapenemase-producing genes began in 2013. Nearly all CPE isolates carried the bla KPC gene, and only 6 isolates carried bla OXA-48 gene, 5 of which were K. pneumoniae and 1 was Citrobacter freundii.
Outbreak description
From January 2016 until May 2017, 658 patients were hospitalized at the ICU, of whom 41 patients (6.4%) acquired CPE. Of these, 31 (76%) acquired OXA-48–producing Enterobacteriaceae and 27 (87%) of these were S. marcescens. Five patients with OXA-48–producing S. marcescens acquired an additional OXA-48–producing Enterobacteriaceae. One patient acquired S. marcescens that harbored both bla OXA-48 and bla KPC genes. Three additional patients were infected with KPC-producing S. marcescens. Patient characterizations of these 34 patients are presented in Table 1. Of 34 cases, 30 were initially detected by routine rectal screening; 11 patients developed clinical infections; and 3 mortality cases were attributed to the infection. Altogether 12 patients died, for an in-hospital mortality rate of 35%. Figure 1 presents the temporal relationship between the outbreak cases, and Figure 2 presents all CPE acquisitions in the ICU during the outbreak year.
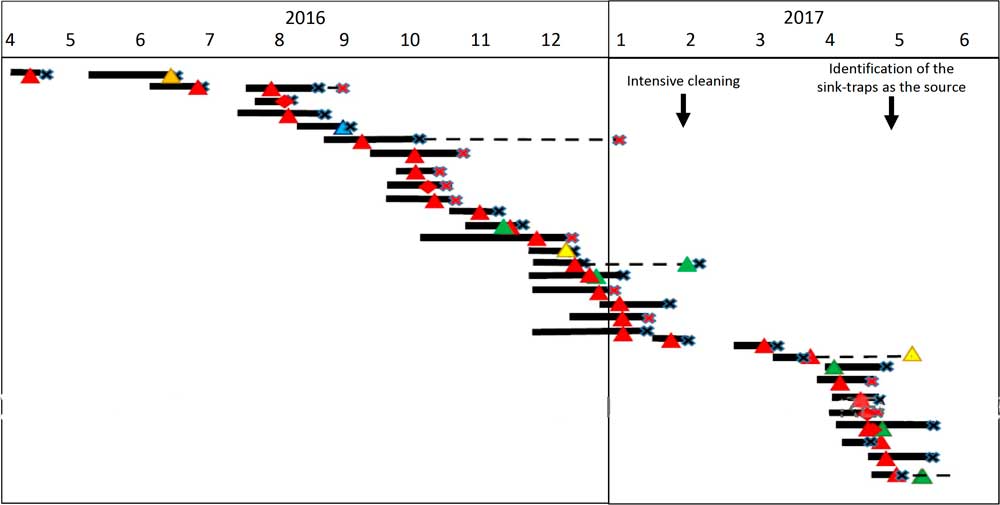
Fig. 1 Synoptic curve of patients colonized and/or infected with OXA-48–producing carbapenemase-producing Enterobacteriaceae (CPE) as well as Klebsiella pneumoniae carbapenemase (KPC)-producing S. marcescens. Black lines denote the duration of hospitalization in the intensive car unity ICU. Dashed lines denote hospitalization outside the ICU that had an unfavorable outcome (mortality or additional CPE acquisition). Note. X denotes the discharge day; red X denotes mortality; triangle denotes acquisition of OXA-48–producing carbapenemase; diamond denotes KPC-producing carbapenemase; red denotes S. marcescens; orange denotes Citerobacter sp; blue denotes E. coli; yellow denotes Enterobacter sp; and green denotes K. pneumoniae.
Table 1 Clinical Characteristics of Patients With OXA-48 Carbapenemase–Producing Enterobacteriaceae (CPE)
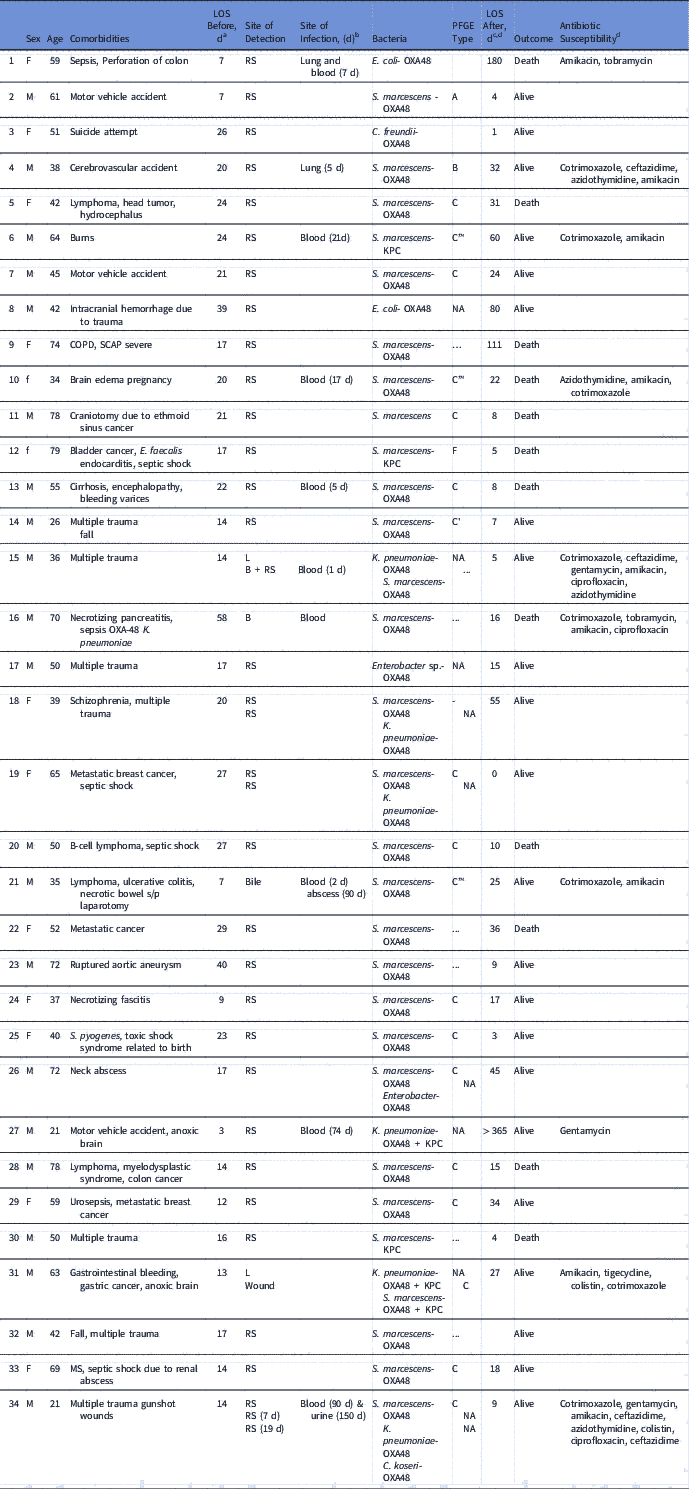
Note. LOS, length of stay; PFGE, pulsed-field gel electrophoresis; RS, rectal swab; COPD, chronic obstructive pulmonary disease NA, nonapplicable (not a Serratia isolate); SCAP, severe community acquired pneumonia; MS, multiple sclerosis.
a Length of stay (days) from admission until CPE was initially detected.
b No. of days after initial CPE detection.
c Length of stay (days) from CPE detection until hospital discharge.
d Antibiotic susceptibility of clinical strains.
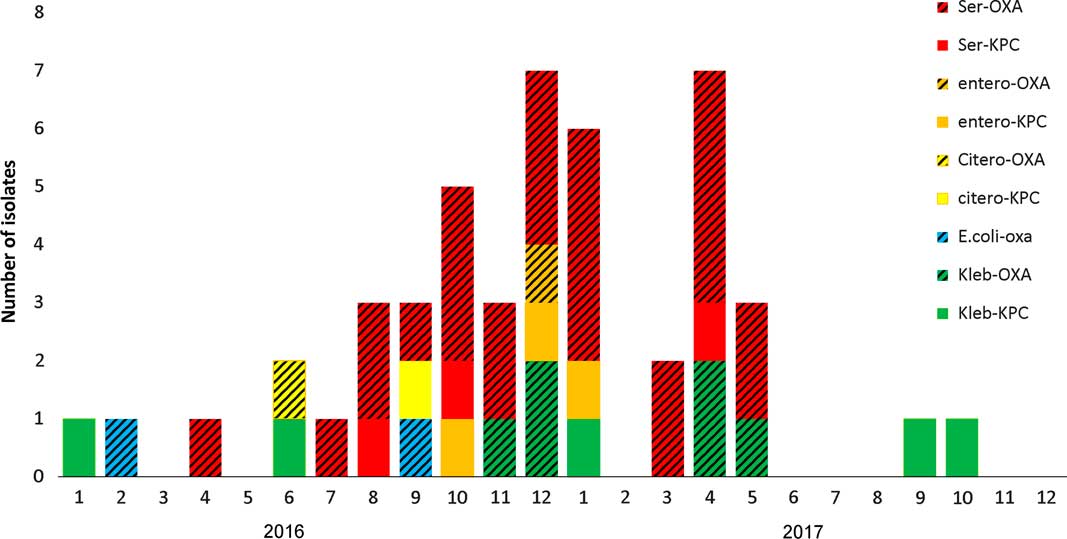
Fig. 2 Carbapenemase-producing Enterobacteriaceae (CPE) acquisitions in the intensive care unit (ICU) during 2016–2017.
Results of the outbreak investigation
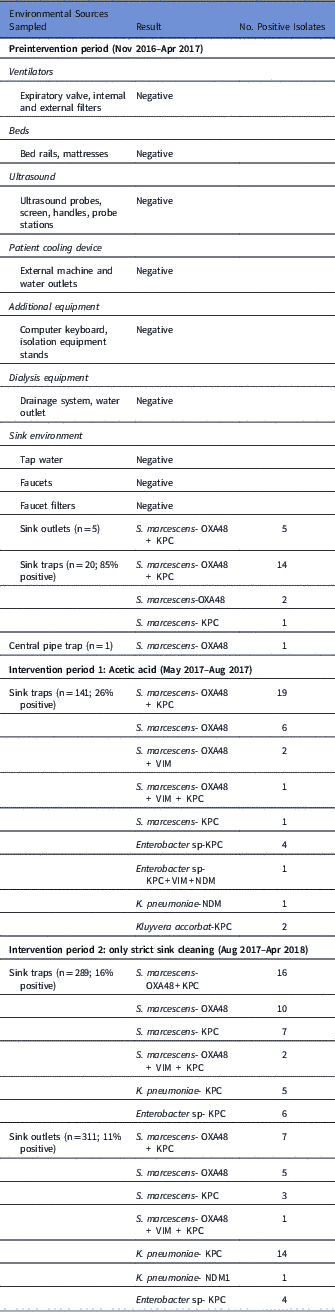
Our initial investigation, including environmental sampling and consultations with the Israeli national infection control unit, did not identify any potential source. The root-cause analysis investigation revealed that significant use of tap water was a common factor in all cases. For most ICU procedures in which water is needed, sterile bottled water is used and tap water is used only for cleaning patients suffering from diarrhea, or rarely when flushing gastric tubes. Sampling of the water environment revealed OXA-48–producing S. marcescens in many sink traps and sink outlets (Table 2).
Table 2 Environmental Samples
Typing S. marcescens isolates
Of the 30 S. marcescens patient isolates, 26 were available and were characterized by PFGE. Apart from the first 2 cases, all OXA-48–producing S. marcescens isolates belonged to a single clone (defined ‘C’). Notably, 1 of the KPC-producing S. marcescens as well as the KPC+OXA-48–producing isolate were nondistinguishable (clone ‘C’). The other available KPC-producing S. marcescens isolate was distinct (defined ‘F’) (Table 1).
Of 101 S. marcescens isolates from the sinks, 57 were available for PFGE. All S. marcescens sink isolates belonged to 2 clones; 38 (67%) were nondistinguishable from the major outbreak clone (‘C’). Most of these (55%) harbored both bla OXA48 and bla KPC genes as detected by PCR, while in 29% only bla OXA48 was detected, in 11% only bla KPC was detected, and in 2 isolates (5%) bla VIM was detected together with bla OXA48+/-bla KPC. The second clone detected in the sinks was nondistinguishable from the KPC-producing S. marcescens of patient 12 (clone ‘F’). Indeed, most of these isolates (63%) harbored only bla KPC, but 7 isolates (37%), harbored bla OXA48+bla KPC.
Control and prevention measures
Following the identification of the sinks, and particularly the sink traps, as the source of transmission, we carried out 2 main measures: (1) sink-trap decontamination efforts and (2) an educational intervention enhancing specific infection control measures and focusing on the sink as a source of transmission. Within a few weeks, bed occupancy returned to full, with 15–16 patients consistently hospitalized in the ICU.
Sink-trap decontamination efforts
Following the identification of the sink traps as a source, all sink traps were replaced, and the water supply was treated according to the Prevention and Control of Legionella Infection Protocol (PCLIP). This intervention consisted of heating and hyperchlorination of the main water tank and terminal points for 12 hours with free residual chlorine (20–30 mg/L). Three days after this procedure, no CPE was detected in any sink trap. However, 10 days later, the outbreak clone was again detected in 14 of 16 sink traps (Figure 3).
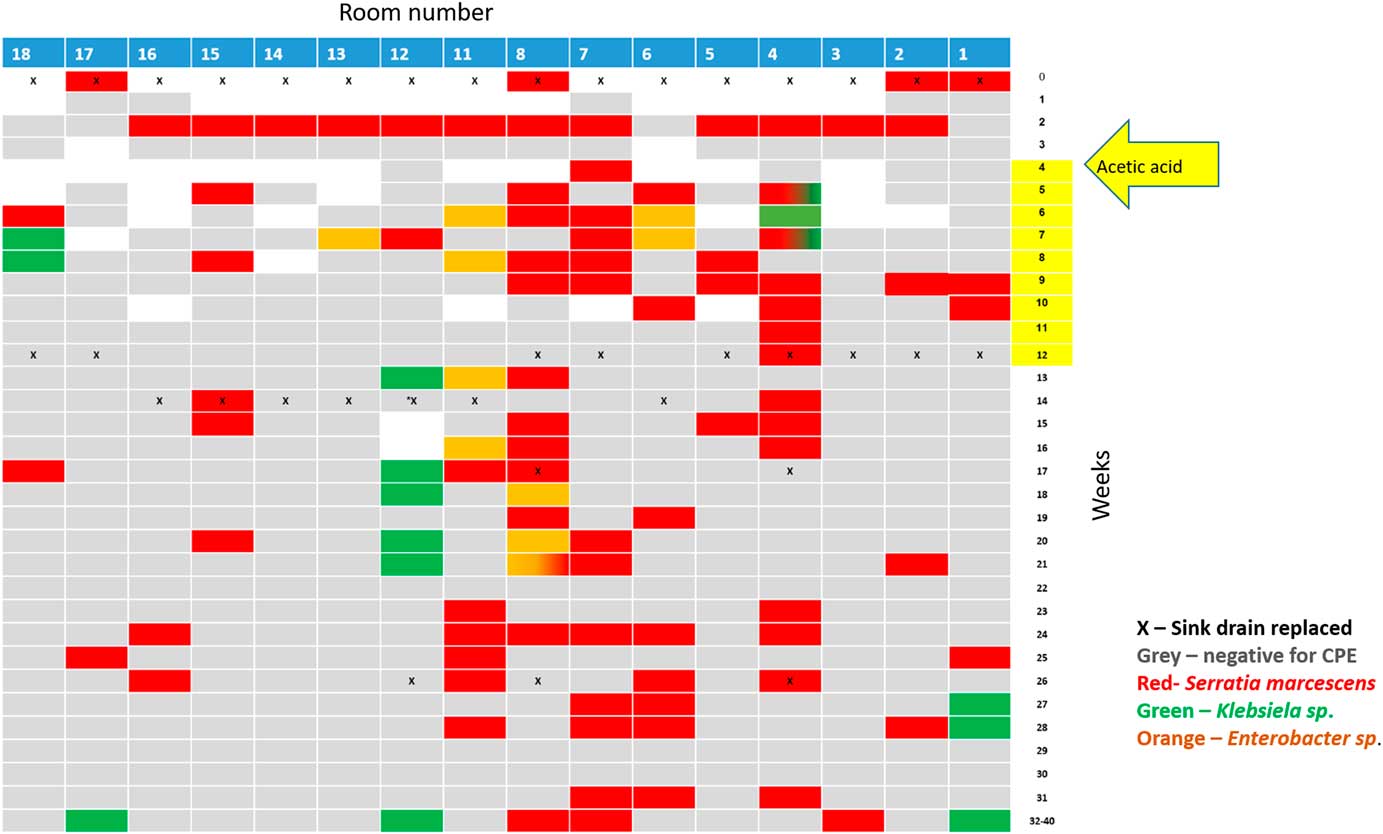
Fig. 3 Results of the weekly surveillance of sink traps in the ICU and the concurrent sink-trap interventions.
Following Stjarne et al,Reference Stjarne Aspelund, Sjostrom, Olsson Liljequist, Morgelin, Melander and Pahlman 9 sink traps were treated with 250 mL 25% acetic acid once weekly for 10 consecutive weeks. Sink traps were also sampled weekly before treatment. This treatment significantly reduced the number of contaminated sinks: 28% versus 85% in the intervention versus preintervention periods (Table 2, Figure 3). Notably, different sinks were contaminated each week, and the staff complained of the odor of acetic acid. On August 3, 2017, this intervention was discontinued. All sink traps were replaced again, and weekly sink-trap sampling continued.
Following Wolf et al,Reference Wolf, Bergervoet, Sebens, van den Oever, Savelkoul and van der Zwet 10 a single ‘self-disinfecting’ sink trap (Medizinische Hygiene-Siphone BIOREC MoveoMed, Dresden, Germany) was purchased (~US$2,300 or ~2000 Euro) and installed in room 12, where a KPC-producing K. pneumoniae had been detected 1 week earlier (Figure 4). This sink trap presumably prevents the formation of a biofilm by means of permanent physical disinfection (heating and ultrasound), electromagnetic cleansing, and antibacterial coating. In the following weeks, the same bacteria continuously grew in the sink outlet despite the experimental sink trap. One month after installation of this sink trap, a patient in room 11 acquired a genetically similar KPC-producing K. pneumoniae. This sink trap was therefore replaced back to a standard sink trap. Currently, a strict daily cleaning protocol with 1,000 ppm hypochlorite is being used, and once-monthly sink-trap surveillance is carried out. The current average proportion of contaminated sinks is ~15% (Table 2).
Educational intervention
A bundled intervention to decrease CPE transmission has been implemented in our institution for a decade as described above. Hand hygiene compliance has been persistently ~80% in the pre-outbreak year. To further engage the ICU team, we conducted 2 workshops, as described above. Following the first workshop, we identified the sink traps as the source of transmission. The second workshop focused on measures to avoid further transmission from contaminated sinks. Strict guidelines on sink use were enforced: (1) using sinks only for hand washing and only when necessary, (2) prohibiting contamination of sinks with clinical waste disposal, and (3) prohibiting storage of materials around the sinks. Adherence to these measures was continuously encouraged by the infection control unit as well as by the ICU team itself, with update presentations, observations, and 10-minute ‘time-out’ sessions.
To further increase awareness and transparency, the number of days without CPE acquisition were posted daily on a centrally located infection control board. On day 100 without CPE acquisition, a celebration was organized with the medical center director, the infection control team, and the ICU teams.
Intervention outcome and follow-up
The last CPE S. marcescens detected in ICU patients, and in general in our medical center, occurred on May 7, 2017, 1 week after the identification of the sink traps as a source of transmission of CPE S. marcescens. Despite continuous growth of carbapenemase-producing S. marcescens in some of the sink traps in the ICU for >11 months, the transmission ceased. Since May 2017, a few CPE-infected patients have been hospitalized in the ICU, but the only 2 CPE acquisitions documented in the ICU were KPC-producing K. pneumoniae, which was probably transmitted from the sink in which the MoveoMed siphon was installed.
Discussion
We describe an OXA-48–producing S. marcescens outbreak in an ICU of a large tertiary-care medical center, and we have demonstrated that the source of transmission was the sink traps. We show that complete elimination of CPE from the sink traps was not successful despite various decontamination approaches, even after nearly 1 year without hospitalized colonized patients as well as several replacements of the sink traps. The outbreak, however, was rapidly contained by a combined intervention: conducting an educational intervention, engaging the team with focus on the proper use of sinks in the ICU, and reducing the environmental contamination load.
Environmental transmission has been considered to have a negligible role in CPE outbreaks and CPE transmission, while patient-to-patient transmission via the hands of healthcare workers has been considered the major route of transmission.Reference Friedman, Carmeli, Walton and Schwaber 7 However, the hospital water environment is increasingly recognized as a potential source of carbapenem-resistant organism (CRO) transmission.Reference Kanamori, Weber and Rutala 11 , Reference Kizny Gordon, Mathers and Cheong 12 . Most reports on CRO outbreaks related to a water reservoir describe Pseudomonas spp or Acinetobacter baumannii outbreaks,Reference Stjarne Aspelund, Sjostrom, Olsson Liljequist, Morgelin, Melander and Pahlman 9 , Reference Kizny Gordon, Mathers and Cheong 12 – Reference Salm, Deja and Gastmeier 14 with only a few CPE outbreaks directly related to water reservoirs, including a KPC-2 producing K. oxytoca,Reference Leitner, Zarfel and Luxner 15 IMP-4-producing Enterobacter cloacae,Reference Betteridge, Merlino, Natoli, Cheong, Gottlieb and Stokes 16 KPC-2–producing K. pneumoniae,Reference Tofteland, Naseer, Lislevand, Sundsfjord and Samuelsen 17 and recently, OXA-48–producing C. freundii.Reference De Geyter, Blommaert and Verbraeken 18
The Serratia species is known to survive and grow in moist environments. Several Serratia outbreaks have been associated with contaminated liquids including saline bottles,Reference Us, Kutlu, Tekeli, Ocal, Cirpan and Memikoglu 19 chlorhexidine,Reference Morillo, Torres, Alonso Salas, Conde and Aznar 20 – Reference de Frutos, Lopez-Urrutia and Dominguez-Gil 22 and IV pain control fluids.Reference Chiang, Wu and Kuo 23 However, the sink environment as a potential source has been suggested only rarely. Ivady et alReference Ivady, Szabo, Damjanova, Pataki, Szabo and Kenesei 24 reported 2 consecutive S. marcescens outbreaks in a NICU where the epidemic strain was detected in 1 culture of a tap water sample between the 2 outbreaks, despite many negative environmental cultures obtained during the outbreaks. Kotsanas et alReference Kotsanas, Wijesooriya and Korman 25 reported the persistence of S. marcescens in several sink traps of an ICU during a CPE outbreak of various species, where 4 of 11 clinical cases were due to IMP-4–producing S. marcescens.
Sink traps have been recently suggested to be a hidden source of gram-negative rod outbreaks in general and CRO in particular.Reference Wolf, Bergervoet, Sebens, van den Oever, Savelkoul and van der Zwet 10 , Reference De Geyter, Blommaert and Verbraeken 18 , Reference Kotsanas, Wijesooriya and Korman 25 – Reference Mathers, Vegesana and German Mesner 27 The mechanism of transmission from sink traps to patients is probably that running water drives aerosol contaminated with bacteria from the biofilm of the sink trap to the sink surrounding. Using a fluorescent marker, it was demonstrated that drain contents splashed at least 1 meter from the sink during hand washing.Reference Hota, Hirji and Stockton 28 , Reference Kotay, Chai, Guilford, Barry and Mathers 29 Furthermore, we observed sterile materials and devices that were about to be inserted into the patients that were misplaced in the very near surrounding of the sinks in the ICU.
The source for the initial OXA-48–producing S. marcescens in our medical center is unclear. Despite rigorous screening for CPE, OXA-48–producing S. marcescens was never detected in our medical center during at least 6 years before the outbreak. A few cases of KPC-producing S. marcescens were previously identified in our ICU as well as a single case of OXA-48–producing K. pneumoniae. As intra- and interpatient dissemination of bla OXA-48 from one species to another has been previously reported.Reference Arana, Saez and Garcia-Hierro 30 Many of the carbapenemase-producing S. marcescens found in the sink traps in our ICU harbored both bla KPC and bla OXA-48, suggesting evolution of multiple CPE in the sink-trap microbiome. Flushing fluids containing antibiotics, including colistin, into the sinks could have further promoted selection of resistant bacteria, particularly Serratia, which are innately resistant to colistin.
The persistence of CPE, and particularly the epidemic strain, for many months after the last infected patient was discharged highlights the importance of this underappreciated environmental reservoir and suggests that the plumbing system is persistently contaminated, even after intensive decontamination efforts including PCIP treatment, weekly treatment with acetic acid, and repeated sink-trap replacement. Indeed, Weingarten et alReference Weingarten, Johnson and Conlan 31 showed that wastewater pipes are a vast resilient reservoir of CPOs; they found common plasmid backbones in both environmental areas and in the patient population. The various infection control strategies to contain outbreaks related to hospital water environment were recently summarized,Reference Kizny Gordon, Mathers and Cheong 12 but none of the strategies reviewed (including bleach, hydrogen peroxide, UV light, or sink and drainage system replacement) was particularly successful, similar to our experience. Especially concerning was the failure of the new and expensive Moveoshiphon technology in our experience. Our result is somewhat unlike that of Mathers et al,Reference Mathers, Vegesana and German Mesner 27 who reported a successful bundled intervention including hopper covers and use of the Moveosiphon. However, they concluded that their ability to assess the efficacy of these self-disinfecting sink traps was limited and requires further evaluation.
While our sink-trap decontamination efforts were not completely successful, they did reduce the number of contaminated sinks and, thus, the overall level of environmental contamination. The second arm of our intervention was an intensive effort to educate the healthcare teams and to engage them in behavioral changes required with regard to the underappreciated contamination risk of the sink surrounding.
It is difficult to measure the role of each of the intervention arms. However, we believe that the team compliance with the new strict sink-use guidelines played a major role in the containment. Engaging teams to a culture change in the field of infection control is extremely complicated, particularly in an ICU, where the treatment of severely acute patients is a high-pressure environment for healthcare workers.
Our study has several limitations. First, we used a 2-arm intervention, and it is impossible to determine the particular role of each arm. Second, the molecular characterization of the isolates was conducted using PFGE. Further analysis using whole-genome sequencing could further clarify the evolution of CPE in sink traps. Last, while we demonstrate the failure of the self-disinfecting sink trap (Moveo), we installed only a single device.
Currently, there seems to be no complete solution for preventing CRO transmission from sink traps to patients. Patient room architecture and sink designs should be carefully conceived. Eliminating sinks in ICU rooms has been suggested,Reference Hopman, Tostmann and Wertheim 32 but this would raise many difficulties because hand washing is frequently necessary, even in the era of alcohol-based hand hygiene, mainly when handling patients with C. difficile infections. A technical plumbing solution, a chemical solution, or an architectural solution should be sought. Meanwhile, healthcare teams should be educated to understand the important role of this hidden reservoir: the sink trap.
Acknowledgments
We thank the ICU team for their cooperation, especially Dr Nir Shimony and Dr David Livingstone for their deep involvement. We thank the Sheba logistic team, particularly the plumbers, Arie Hikrey and Avi Simchi, for their assistance. We thank the Infection Prevention and Control team for their dedicated work, and Aylana Reiss-Mandel for her assistance in the laboratory work, as well as Ronen Gellstein, Raya Bornstein, and Iris Stein from Magen Haim for their continuous support.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.









