Media summary: Cultural evolution theory has shown that behaviours that are learnt socially or culturally – rather than inherited genetically – can evolve in a Darwinian fashion. Two selective processes can potentially lead to a culturally transmitted behaviour becoming widespread. In Cultural Selection 1, a behaviour is selected because it results in an individual having more children. Instead, in Cultural Selection 2, it is selected because it results in more ‘cultural children’, that is more learners or apprentices. I argue that distinguishing between these two processes is crucial and that building mathematical models of Cultural Selection 1 can help the advancement of cultural evolution theory.
Smith (Reference Smith2020) provides an insightful and balanced review of cultural group selection (CGS) theory. While underlining the merits of the theory, he also highlights several difficulties with it, which mean that the explanation for human social evolution offered by CGS in its current form is not entirely satisfactory. One such difficulty is the theory's inability to clearly separate the effects of selection of cultural traits based on an individual's biological fitness (i.e. number of offspring) from the effects of selection on her cultural fitness (i.e. number of apprentices/learners) (Smith Reference Smith2020, pp. 17–18,22). As these two processes – recently termed Cultural Selection 1 (CS1) and Cultural Selection 2 (CS2), respectively (Birch Reference Birch2017) – can lead to different evolutionary outcomes, failing to distinguish between them can cause confusion (see Fig. 1 for an illustration).
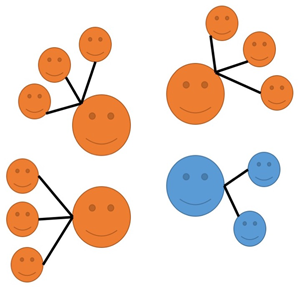
Figure 1. Cultural Selection 1 (CS1) and Cultural Selection 2 (CS2): an illustration. In both processes, orange icons represent individuals expressing cultural variant A, and blue icons represent individuals expressing a different cultural variant, B, for a given cultural trait (grey icons represent juveniles who do not have a cultural variant for the trait yet). In CS1: (1) adults reproduce asexually, with Oranges having higher fertility than Blues thanks to their cultural variant, and they transmit their cultural variant to their offspring; (2) unbiased horizontal transmission of cultural variants occurs between juveniles; (3) density-dependent regulation occurs, with a limited number of randomly chosen juveniles reaching adulthood. In CS2: (1) adults reproduce asexually, with Oranges and Blues having equal fertility, and their offspring not having either cultural variant for the trait at the start; (2) vertical and oblique transmission of cultural variants occurs, with juveniles learning a cultural variant from adults and with Oranges having a greater ability to attract learners than Blues thanks to their cultural variant; (3) density-dependent regulation occurs, with a limited number of randomly chosen juveniles reaching adulthood. In both cases, effects of random sampling are ignored to better illustrate the action of the two selective processes. Notice that these are just two examples (making specific assumptions about cultural transmission) and they are simply meant to illustrate how CS1 and CS2 can operate.
Moreover, I believe that lack of clarity concerning CS1 and CS2 – in the context of CGS and cultural evolution more in general – can hinder the integration of explanations based on social learning and adaptation to different ecologies. Recent empirical work has made some progress towards such an integration (Botero et al. Reference Botero, Gardner, Kirby, Bulbulia, Gavin and Gray2014; Colleran Reference Colleran2016; Mattison et al. Reference Mattison, Moya, Reynolds and Towner2018). Here, I suggest that mathematical modelling of human social evolution can move in the same direction by further developing models of CS1. This should be done by building on existing social evolution theory methodologies and replacing genetic relatedness with cultural relatedness, i.e. the probability that individuals share a cultural variant (Allison Reference Allison1992; Lehmann et al. Reference Lehmann, Feldman and Foster2008; Bell et al. Reference Bell, Richerson and McElreath2009; Boyd et al. Reference Boyd, Richerson and Henrich2011; Birch Reference Birch2017; Handley and Mathew Reference Handley and Mathew2020).
Human behaviours are influenced by both genetically inherited factors (genes) and culturally inherited factors, which may follow different evolutionary trajectories. Social evolution theory in biology has revealed that genetic natural selection tends to lead to adaptive behaviours that maximize an individual's inclusive fitness, i.e. her own reproduction and that of social partners, weighted by her genetic relatedness to them (Hamilton Reference Hamilton1964; Grafen Reference Grafen2006; Gardner Reference Gardner2009; Lehmann and Rousset Reference Lehmann and Rousset2020). Genetic relatedness measures the probability that two individuals share a given gene, relative to the population average, and generally coincides with genealogical kinship (Frank Reference Frank1998). Human behavioural ecology has applied inclusive fitness theory to the study of human behaviour with considerable success. However, this approach has tended to consider culture only as a proximate mechanism that does not to pose constraints on evolution (the ‘phenotypic gambit’; Nettle et al. Reference Nettle, Gibson, Lawson and Sear2013).
Yet should we expect the evolution of culturally inherited traits to lead to the same adaptive outcomes as the evolution of genetically inherited ones? To answer this question, it is crucial to distinguish between CS1 and CS2. In recent years, cultural selection on cultural fitness (CS2) has received considerable attention, with several suggestions that this process can lead to biologically maladaptive traits (e.g. Henrich Reference Henrich2004a; Richerson and Boyd Reference Richerson and Boyd2005; Mesoudi et al. Reference Mesoudi, Whiten and Laland2006; Tanaka et al. Reference Tanaka, Kendal and Laland2009). CGS has played an important role in these efforts, because selection of group-level cultural traits through between-group competition always involves CS2, but not always CS1, as Smith (Reference Smith2020, p. 18) clarifies.
On the other hand, CS1 has been relatively neglected. In this selective process, the currency is the same as in genetic evolution, but the transmission of the cultural variants determining the behaviour is potentially different, because they can be inherited vertically, obliquely (from a non-parental adult), horizontally (within a generation) or in a combination of these modes (Birch Reference Birch2017). Thus, rather than genetic relatedness, we need to consider cultural relatedness, that is the probability that two individuals share a cultural variant (Allison Reference Allison1992; Lehmann et al. Reference Lehmann, Feldman and Foster2008; Bell et al. Reference Bell, Richerson and McElreath2009; Boyd et al. Reference Boyd, Richerson and Henrich2011; Birch Reference Birch2017; Handley and Mathew Reference Handley and Mathew2020). For a given trait, cultural relatedness can in principle be higher, lower or equal to genetic relatedness. If horizontal and oblique transmission are unbiased, then the qualitative predictions of a CS1 model – for example, regarding the direction of sex differences in behaviour – would be the same as for a genetic model. However, predictions might differ quantitatively – i.e. the magnitude of those sex differences might differ – because cultural relatedness can be higher than genetic relatedness (Lehmann et al. Reference Lehmann, Feldman and Foster2008; Micheletti et al. Reference Micheletti, Ruxton and Gardner2020).
Some steps in the analysis of CS1 have been taken. Lehmann et al. (Reference Lehmann, Feldman and Foster2008) and Lehmann and Feldman (Reference Lehmann and Feldman2008) have shown that altruism can evolve more or less readily under CS1 than genetic selection depending on social learning modes (but see Boyd et al. Reference Boyd, Richerson and Henrich2011). In addition, Birch (Reference Birch2017) has suggested that CS1 might have driven the evolution of prosocial tendencies and has proposed an expanded definition of relatedness that captures horizontal transmission. Notwithstanding this work, CS1 is generally not being employed to model specific questions of human social evolution. To encourage this, methods to build CS1 models should be presented in an accessible format, which clarifies the key assumptions concisely and includes some worked examples (e.g. modelled after Taylor and Frank Reference Taylor and Frank1996). Moreover, more formal theory should be developed to analyse the effects of different forms of transmission (e.g. including non-vertical transmission, biased or unbiased; starting from Lehmann et al. Reference Lehmann, Feldman and Foster2008; Lehmann and Feldman Reference Lehmann and Feldman2008; Boyd et al. Reference Boyd, Richerson and Henrich2011; Birch Reference Birch2017) and explore interaction with CS2 processes (starting from Henrich Reference Henrich2004b; El Mouden et al. Reference El Mouden, André, Morin and Nettle2014).
In conclusion, modelling CS1 – selection of cultural variants based on their effects on biological fitness – can act as a bridge between social learning explanations, which often focus on cultural transmission, and adaptive explanations, centred on inclusive fitness. Moreover, employing CS1 models, instead of genetic ones, would greatly facilitate interaction between empiricists and theoreticians, especially for behaviours that have a clear and strong culturally inherited component (e.g. post-marital residence). Importantly, such efforts would not necessitate the development of models ex novo. Instead, they should build upon the already advanced mathematical machinery of genetic social evolution theory (Frank Reference Frank1998; Rousset Reference Rousset2004). Thus, this new class of models could be intended as a ‘cultural’ expansion of the Hamilton's rule organising framework (Birch Reference Birch2017), creating a natural bridge between inclusive fitness and cultural evolution approaches.






Media summary: Cultural evolution theory has shown that behaviours that are learnt socially or culturally – rather than inherited genetically – can evolve in a Darwinian fashion. Two selective processes can potentially lead to a culturally transmitted behaviour becoming widespread. In Cultural Selection 1, a behaviour is selected because it results in an individual having more children. Instead, in Cultural Selection 2, it is selected because it results in more ‘cultural children’, that is more learners or apprentices. I argue that distinguishing between these two processes is crucial and that building mathematical models of Cultural Selection 1 can help the advancement of cultural evolution theory.
Smith (Reference Smith2020) provides an insightful and balanced review of cultural group selection (CGS) theory. While underlining the merits of the theory, he also highlights several difficulties with it, which mean that the explanation for human social evolution offered by CGS in its current form is not entirely satisfactory. One such difficulty is the theory's inability to clearly separate the effects of selection of cultural traits based on an individual's biological fitness (i.e. number of offspring) from the effects of selection on her cultural fitness (i.e. number of apprentices/learners) (Smith Reference Smith2020, pp. 17–18,22). As these two processes – recently termed Cultural Selection 1 (CS1) and Cultural Selection 2 (CS2), respectively (Birch Reference Birch2017) – can lead to different evolutionary outcomes, failing to distinguish between them can cause confusion (see Fig. 1 for an illustration).
Figure 1. Cultural Selection 1 (CS1) and Cultural Selection 2 (CS2): an illustration. In both processes, orange icons represent individuals expressing cultural variant A, and blue icons represent individuals expressing a different cultural variant, B, for a given cultural trait (grey icons represent juveniles who do not have a cultural variant for the trait yet). In CS1: (1) adults reproduce asexually, with Oranges having higher fertility than Blues thanks to their cultural variant, and they transmit their cultural variant to their offspring; (2) unbiased horizontal transmission of cultural variants occurs between juveniles; (3) density-dependent regulation occurs, with a limited number of randomly chosen juveniles reaching adulthood. In CS2: (1) adults reproduce asexually, with Oranges and Blues having equal fertility, and their offspring not having either cultural variant for the trait at the start; (2) vertical and oblique transmission of cultural variants occurs, with juveniles learning a cultural variant from adults and with Oranges having a greater ability to attract learners than Blues thanks to their cultural variant; (3) density-dependent regulation occurs, with a limited number of randomly chosen juveniles reaching adulthood. In both cases, effects of random sampling are ignored to better illustrate the action of the two selective processes. Notice that these are just two examples (making specific assumptions about cultural transmission) and they are simply meant to illustrate how CS1 and CS2 can operate.
Moreover, I believe that lack of clarity concerning CS1 and CS2 – in the context of CGS and cultural evolution more in general – can hinder the integration of explanations based on social learning and adaptation to different ecologies. Recent empirical work has made some progress towards such an integration (Botero et al. Reference Botero, Gardner, Kirby, Bulbulia, Gavin and Gray2014; Colleran Reference Colleran2016; Mattison et al. Reference Mattison, Moya, Reynolds and Towner2018). Here, I suggest that mathematical modelling of human social evolution can move in the same direction by further developing models of CS1. This should be done by building on existing social evolution theory methodologies and replacing genetic relatedness with cultural relatedness, i.e. the probability that individuals share a cultural variant (Allison Reference Allison1992; Lehmann et al. Reference Lehmann, Feldman and Foster2008; Bell et al. Reference Bell, Richerson and McElreath2009; Boyd et al. Reference Boyd, Richerson and Henrich2011; Birch Reference Birch2017; Handley and Mathew Reference Handley and Mathew2020).
Human behaviours are influenced by both genetically inherited factors (genes) and culturally inherited factors, which may follow different evolutionary trajectories. Social evolution theory in biology has revealed that genetic natural selection tends to lead to adaptive behaviours that maximize an individual's inclusive fitness, i.e. her own reproduction and that of social partners, weighted by her genetic relatedness to them (Hamilton Reference Hamilton1964; Grafen Reference Grafen2006; Gardner Reference Gardner2009; Lehmann and Rousset Reference Lehmann and Rousset2020). Genetic relatedness measures the probability that two individuals share a given gene, relative to the population average, and generally coincides with genealogical kinship (Frank Reference Frank1998). Human behavioural ecology has applied inclusive fitness theory to the study of human behaviour with considerable success. However, this approach has tended to consider culture only as a proximate mechanism that does not to pose constraints on evolution (the ‘phenotypic gambit’; Nettle et al. Reference Nettle, Gibson, Lawson and Sear2013).
Yet should we expect the evolution of culturally inherited traits to lead to the same adaptive outcomes as the evolution of genetically inherited ones? To answer this question, it is crucial to distinguish between CS1 and CS2. In recent years, cultural selection on cultural fitness (CS2) has received considerable attention, with several suggestions that this process can lead to biologically maladaptive traits (e.g. Henrich Reference Henrich2004a; Richerson and Boyd Reference Richerson and Boyd2005; Mesoudi et al. Reference Mesoudi, Whiten and Laland2006; Tanaka et al. Reference Tanaka, Kendal and Laland2009). CGS has played an important role in these efforts, because selection of group-level cultural traits through between-group competition always involves CS2, but not always CS1, as Smith (Reference Smith2020, p. 18) clarifies.
On the other hand, CS1 has been relatively neglected. In this selective process, the currency is the same as in genetic evolution, but the transmission of the cultural variants determining the behaviour is potentially different, because they can be inherited vertically, obliquely (from a non-parental adult), horizontally (within a generation) or in a combination of these modes (Birch Reference Birch2017). Thus, rather than genetic relatedness, we need to consider cultural relatedness, that is the probability that two individuals share a cultural variant (Allison Reference Allison1992; Lehmann et al. Reference Lehmann, Feldman and Foster2008; Bell et al. Reference Bell, Richerson and McElreath2009; Boyd et al. Reference Boyd, Richerson and Henrich2011; Birch Reference Birch2017; Handley and Mathew Reference Handley and Mathew2020). For a given trait, cultural relatedness can in principle be higher, lower or equal to genetic relatedness. If horizontal and oblique transmission are unbiased, then the qualitative predictions of a CS1 model – for example, regarding the direction of sex differences in behaviour – would be the same as for a genetic model. However, predictions might differ quantitatively – i.e. the magnitude of those sex differences might differ – because cultural relatedness can be higher than genetic relatedness (Lehmann et al. Reference Lehmann, Feldman and Foster2008; Micheletti et al. Reference Micheletti, Ruxton and Gardner2020).
Some steps in the analysis of CS1 have been taken. Lehmann et al. (Reference Lehmann, Feldman and Foster2008) and Lehmann and Feldman (Reference Lehmann and Feldman2008) have shown that altruism can evolve more or less readily under CS1 than genetic selection depending on social learning modes (but see Boyd et al. Reference Boyd, Richerson and Henrich2011). In addition, Birch (Reference Birch2017) has suggested that CS1 might have driven the evolution of prosocial tendencies and has proposed an expanded definition of relatedness that captures horizontal transmission. Notwithstanding this work, CS1 is generally not being employed to model specific questions of human social evolution. To encourage this, methods to build CS1 models should be presented in an accessible format, which clarifies the key assumptions concisely and includes some worked examples (e.g. modelled after Taylor and Frank Reference Taylor and Frank1996). Moreover, more formal theory should be developed to analyse the effects of different forms of transmission (e.g. including non-vertical transmission, biased or unbiased; starting from Lehmann et al. Reference Lehmann, Feldman and Foster2008; Lehmann and Feldman Reference Lehmann and Feldman2008; Boyd et al. Reference Boyd, Richerson and Henrich2011; Birch Reference Birch2017) and explore interaction with CS2 processes (starting from Henrich Reference Henrich2004b; El Mouden et al. Reference El Mouden, André, Morin and Nettle2014).
In conclusion, modelling CS1 – selection of cultural variants based on their effects on biological fitness – can act as a bridge between social learning explanations, which often focus on cultural transmission, and adaptive explanations, centred on inclusive fitness. Moreover, employing CS1 models, instead of genetic ones, would greatly facilitate interaction between empiricists and theoreticians, especially for behaviours that have a clear and strong culturally inherited component (e.g. post-marital residence). Importantly, such efforts would not necessitate the development of models ex novo. Instead, they should build upon the already advanced mathematical machinery of genetic social evolution theory (Frank Reference Frank1998; Rousset Reference Rousset2004). Thus, this new class of models could be intended as a ‘cultural’ expansion of the Hamilton's rule organising framework (Birch Reference Birch2017), creating a natural bridge between inclusive fitness and cultural evolution approaches.
Acknowledgements
I thank Francesca De Petrillo, Maxime Derex, Charles Efferson, Rebecca Feng, Armando Micheletti, Catherine Molho, Jorge Peña and Jonathan Stieglitz for insightful comments on previous versions of this paper, and Jonathan Birch for a useful discussion on cultural evolution.
Financial support
Funding from the French Agence Nationale de la Recherche (under the Investissement d'Avenir programme, ANR 17-EURE-0010) is gratefully acknowledged.
Publishing ethics
The manuscript is my own original work, and does not duplicate any other previously published work. The manuscript has been submitted only to the journal – it is not under consideration, accepted for publication or in press elsewhere. All listed authors know of and agree to the manuscript being submitted to the journal. The manuscript contains nothing that is abusive, defamatory, fraudulent, illegal, libellous or obscene.
Conflict of interest
There are no conflicts of interest.