Mental health problems affect 10–20% of children and adolescents worldwide and are associated with adverse health outcomes in the short and long terms (Kieling et al., Reference Kieling, Baker-Henningham, Belfer, Conti, Ertem, Omigbodun, Rohde, Srinath, Ulkuer and Rahman2011; Prince et al., Reference Prince, Patel, Saxena, Maj, Maselko, Phillips and Rahman2007). Behavior disorders, often diagnosed in adolescence (Patton et al., Reference Patton, Sawyer, Santelli, Ross, Afifi, Allen, Arora, Azzopardi, Baldwin, Bonell, Kakuma, Kennedy, Mahon, McGovern, Mokdad, Patel, Petroni, Reavley, Taiwo and Viner2016), substantially contribute to disability, low quality of life, and early mortality (Kessler et al., Reference Kessler, Angermeyer, Anthony, de Graaf, Demyttenaere, Gasquet, de Girolamo, Gluzman, Gureje, Haro, Kawakami, Karam, Levinson, Medina Mora, Oakley Browne, Posada-Villa, Stein, Adley Tsang, Aguilar-Gaxiola and Ustün2007; Roza et al., Reference Roza, Hofstra, Ende and Verhulst2003). Elucidating the determinants of behavior problems in adolescence could contribute to the prevention of future severe disorders.
Sleep is vital for the restitution and regulation of many systems in the body. Despite the importance of adequate sleep during development, approximately one-third of children experience inadequate sleep duration (Wheaton & Claussen, Reference Wheaton and Claussen2021). Insufficient and excessive sleep are associated with nervous system activation, which may result in increased blood pressure and cortisol secretion, metabolic disorders, and immune dysfunction (Itani et al., Reference Itani, Jike, Watanabe and Kaneita2017; Jike et al., Reference Jike, Itani, Watanabe, Buysse and Kaneita2018). Sleep also plays an important role in regulating emotion and behavior in children. Sleep deprivation can result in poor memory consolidation, poor cognitive performance, and mood changes in adults (Alhola & Polo-Kantola, Reference Alhola and Polo-Kantola2007). It impairs self-regulation abilities and social monitoring which can lead to emotional disinhibition, increased response to negative stimuli; lack of empathy, humor, and ability to trust; and rule-breaking behaviors (Gujar et al., Reference Gujar, Yoo, Hu and Walker2011).
Middle childhood is a critical period in cognitive and emotional development because the prefrontal cortex, a region of the brain that is central to these functions, matures slowly. This region may be very sensitive to sleep disruptions (Dahl, Reference Dahl1996; Walker, Reference Walker2009); and sleep-related alterations in brain development during critical periods could result in the development of behavior problems. Since many behavior problems become manifest during adolescence, middle childhood may represent a valuable window for early intervention.
Previous epidemiologic studies have shown an association between short sleep duration and behavior problems in both adults (Dinges et al., Reference Dinges, Pack, Williams, Gillen, Powell, Ott, Aptowicz and Pack1997) and children (Gregory et al., Reference Gregory, van der Ende, Willis and Verhulst2008; Nixon et al., Reference Nixon, Thompson, Han, Becroft, Clark, Robinson, Waldie, Wild, Black and Mitchell2008; Ranum et al., Reference Ranum, Wichstrøm, Pallesen, Falch-Madsen, Halse and Steinsbekk2019). An association with long sleep duration is also plausible (James & Hale, Reference James and Hale2017a; Zhai et al., Reference Zhai, Zhang and Zhang2015), but most investigations have failed to characterize an oversleeping exposure group (Weaver et al., Reference Weaver, Barger, Malone, Anderson and Klerman2018). In addition, the association between sleep in middle childhood, a critical neurodevelopmental period, and behavior in adolescence is still unclear.
The objective of this study was to assess the association between middle childhood sleep duration and the development of behavior problems in adolescence using data from the Bogotá School Children Cohort (BoSCCo) study. A secondary aim was to examine the associations of sleep duration in adolescence with behavior problems.
Method
Study design and population
We conducted a prospective investigation in the context of the BoSCCo study. Details on the cohort design have been previously reported (Arsenault et al., Reference Arsenault, Mora-Plazas, Forero, López-Arana, Marín, Baylin and Villamor2009; Robinson et al., Reference Robinson, Marín, Oliveros, Mora-Plazas, Richards, Lozoff and Villamor2018). In brief, we recruited 3,202 randomly selected children aged 5–12 years in February 2006 from primary public schools in Bogotá, Colombia. These schools enrolled children of low- and middle-income backgrounds; thus, the sample pertains to these groups.
Baseline information
At the time of enrollment, parents completed a self-administered survey that included information on children’s behaviors and habits; maternal parity and anthropometry; and household characteristics including socioeconomic status as categorized by the local government, and level of food insecurity per a validated Spanish version of the US Department of Agriculture Household Food Security Survey module (Harrison et al., Reference Harrison, Stormer, Herman and Winham2003).
During the weeks following enrollment, research assistants visited the schools to perform anthropometry on the children. Height was measured without shoes to the nearest 1 mm using portable wall-mounted Seca 202 stadiometers (Seca Hanover, MD) and weight was measured in light clothing to the nearest 0.1 kg using Tanita H5301 electronic scales (Tanita, Arlington Heights, IL). At the end of the visit, researchers obtained a fasting blood sample via antecubital venipuncture; aliquots were collected in EDTA-coated tubes and metal-free polypropylene tubes without anticoagulant for separation of serum. Samples were protected from sunlight and placed in refrigerated coolers for transportation to the Colombian National Institute of Health on the day of collection, where they were processed and cryostored.
Between 2007 and 2009, parents of 2308 randomly selected participants completed a follow-up self-administered survey that included questions on the children’s nighttime sleep duration on weekdays and weekend days. The question for weekdays was: “Normally, how many hours does the child sleep at night during weekdays? (count the hours and minutes from the time the child falls asleep at night to the time the child awakens in the morning)”, with fields for duration in hours and minutes. A second question replaced “weekdays” with “weekend days”. The questionnaire also included separate questions on whether the child had ever been diagnosed by a physician with asthma or allergies.
Adolescence follow-up
Between 2011 and 2015, an in-person follow-up assessment was conducted in approximately one-third of the cohort, chosen through random sampling (n = 1,139). These assessments occurred primarily at schools, or at home if the participant was absent from school. Parents completed a self-administered survey that updated baseline information on children’s behaviors and habits; maternal parity and anthropometry; and household characteristics. Bedtimes and waketimes were assessed through an adolescent self-administered survey with separate questions for weekdays and weekend days. The question for weekdays was: “Normally, at what time do you fall asleep at night during weekdays”, with fields for time in hours and minutes. A separate question replaced “weekdays” with “weekend days”.
We assessed adolescence behavior problems using the Spanish language versions of the Youth Self-Report (YSR) and the Child Behavior Checklist (CBCL), which were completed by the child and the parent, respectively. Each instrument consists of 112 statements on behaviors and feelings that the respondents may mark as false, sometimes true, or very/often true. Using these responses, software provided by the test developers computes continuous scores in eight behavior problem subscales (Achenbach, Reference Achenbach2000), standardized by age and sex from a reference US population. The subscales include aggressive, rule-breaking, anxious/depressed, and withdrawn/depressed behaviors, somatic complaints, and attention, social, and thought problems. The software also provides composite scores for externalizing and internalizing problems. Total externalizing problems comprise the aggressive and rule-breaking behavior subscales while total internalizing problems consist of the anxious/depressed, withdrawn/depressed, and somatic complaints subscales. The YSR has been validated for use in adolescents aged 11–18 years and the CBCL has been validated in children aged 5–18 years (Achenbach & Rescorla, Reference Achenbach and Rescorla2001). Both instruments have been widely administered in Latin American settings (Rescorla et al., Reference Rescorla, Ivanova, Achenbach, Begovac, Chahed, Drugli, Emerich, Fung, Haider, Hansson, Hewitt, Jaimes, Larsson, Maggiolini, Marković, Mitrović, Moreira, Oliveira, Olsson and Zhang2012).
Written informed consent was obtained from the primary care providers prior to enrollment. The children provided assent to participate. The study protocol was approved by the Ethics Committee of the National University of Colombia Medical School. The University of Michigan Institutional Review Board approved the use of data from the study.
Laboratory methods
All laboratory analyses were conducted at the Colombian National Institute of Health in Bogotá. Plasma ferritin and vitamin B12 were quantified through competitive chemiluminescent immunoassay in an ADVIA Centaur analyzer (Bayer Diagnostics, Tarrytown, NY). Serum C-reactive protein (CRP) concentration was measured with the use of a turbidimetric immunoassay on an ACS180 analyzer (Bayer Diagnostics, Tarrytown, NY). Hemoglobin concentrations were determined by the hemoglobincyanide method.
Data analysis
Outcomes
The primary outcomes of interest were total externalizing and internalizing behavior scores. Secondary outcomes were the eight behavior problem subscales.
Exposure
The primary exposure was nighttime sleep duration in middle childhood. Sleep duration was calculated as the weighted average of the weekday (weight, 5/7) and weekend day (weight, 2/7) reported sleep hours. Nighttime sleep duration in hours and minutes was categorized per the American Academy of Sleep Medicine age-specific recommendations (Paruthi et al., Reference Paruthi, Brooks, D’Ambrosio, Hall, Kotagal, Lloyd, Malow, Maski, Nichols, Quan, Rosen, Troester and Wise2016) as under, within, or above. Cut points (hours) for each group were, respectively, <9, 9–12, or >12 for children 6–12 year-old, and <8, 8–10, or >10 for those aged ≥13 years. The secondary exposure, sleep duration in adolescence, was estimated in decimal hours as the difference between waketime and bedtime and categorized using the same age-specific recommendations. Both exposures were also considered as continuous variables.
Covariates
Children’s height- and body mass index (BMI, kg/m2)-for-age Z scores at enrollment were calculated according to the WHO growth reference for children and adolescents (de Onis et al., Reference de Onis, Onyango, Borghi, Siyam, Nishida and Siekmann2007). Screen time was the sum of weekly hours spent watching television or playing video games as reported at baseline. Maternal education was the number of years of formal schooling and parity was the number of previous live births. Household food insecurity was defined as severe when participants responded affirmatively to ≥13 of the survey’s 16 questions on adverse experiences (Harrison et al., Reference Harrison, Stormer, Herman and Winham2003). Socioeconomic status was categorized from 1 (lowest) to 4 (highest in the sample). Anemia was defined as hemoglobin <12.7 g/dL, an altitude-adjusted cut point (World Health Organization, 2011). Iron deficiency was defined as plasma ferritin <15 μg/L in children with CRP <10 mg/L, and low vitamin B12 serostatus was defined as concentrations (pmol/L) below the lowest quartile of the sex-specific distributions, 247.5 in boys and 261.5 in girls (Robinson et al., Reference Robinson, Marín, Oliveros, Mora-Plazas, Richards, Lozoff and Villamor2018).
Statistical analysis
Of 1139 participants followed into adolescence, 1084 and 863 had valid YSR and CBCL assessments, respectively. We excluded 42 YSR and 9 CBCL assessments that were outside their corresponding valid age ranges. Of the remaining 1061 participants with either YSR or CBCL valid evaluations, 889 had nighttime sleep duration data in middle childhood, and these constituted the analytic sample (872 for YSR and 729 for CBCL).
We first compared the distributions of background characteristics by categories of middle childhood nighttime sleep duration to identify potential confounders. Next, we compared the continuous distributions of total externalizing and internalizing behavior problem scores and their subscales between middle childhood sleep duration categories using means and SD and tested the statistical significance of differences with use of the Kruskal–Wallis test. We then estimated adjusted mean differences with 95% confidence intervals (CI) between under or above sleep duration recommendations compared with within recommendations, using multiple linear regression models. Adjustment variables included baseline sociodemographic and behavioral characteristics that were related to the exposure but were not its consequence, or that were known independent predictors of behavior problems (Parent et al., Reference Parent, Sanders and Forehand2016). These included child’s sex, baseline age, and screen time; and SES indicators such as mother’s education and parity, and household food insecurity. Screentime is an independent predictor of externalizing behavior in this population (Robinson et al., Reference Robinson, Marín, Oliveros, Mora-Plazas, Richards, Lozoff and Villamor2018) and a predictor of sleep duration (Guerrero et al., Reference Guerrero, Barnes, Chaput and Tremblay2019). To ascertain whether the associations of middle childhood sleep duration with behavior problems were independent of adolescence sleep duration, we adjusted for adolescence sleep in the main models. Because adolescence sleep could be a mediator between middle childhood sleep duration and behavior outcomes, the adjusted estimate would correspond to the direct effect of middle childhood sleep under the assumptions of a counterfactual framework (Valeri & VanderWeele, Reference Valeri and VanderWeele2013).
We performed further adjustment for child nutrition factors that have been related to behavior problems in this population, including middle-childhood height and BMI-for-age Z scores, anemia, iron deficiency, and low vitamin B12 serostatus (Robinson et al., Reference Robinson, Marín, Oliveros, Mora-Plazas, Richards, Lozoff and Villamor2018). Micronutrient biomarkers were only assessed at the time of enrollment, approximately 2 years prior to the ascertainment of sleep duration. Notwithstanding, since these biomarkers could reflect long-term status (Gibson, Reference Gibson2005), their inclusion as adjustment variables, even if assessed at a time prior to exposure, is warranted for control of potential confounding. We also adjusted for having had diagnoses of asthma or allergies, which could affect sleep duration and be related to behavior outcomes (Xie et al., Reference Xie, Kakkar, Teodorescu, Herpel, Krishnan and Teodorescu2010). Because up to 13% of values on some of the nutritional covariates were missing (Supplemental Table 1), we conducted this supplemental adjustment with use of multiple imputation. We generated 10 datasets with predicted missing values through a Markov Chain Monte Carlo procedure in which all available variables were entered regardless of their temporal relations (Schaefer, Reference Schaefer1997). Multivariable-adjusted models were run on each imputed dataset and the estimates were combined across models using Rubin’s rules (Rubin, Reference Rubin1987).
We examined whether the associations differed by sex through stratification since the effects of sleep on health outcomes may vary by sex (Leadbeater et al., Reference Leadbeater, Kuperminc, Blatt and Hertzog1999). Interaction terms between sex and sleep duration categories were tested with use of χ2 Score tests. We also explored whether associations differed for weekday vs. weekend sleep duration following similar methods. These supplemental analyses were restricted to outcomes pertaining to the YSR, due to small sample sizes with the CBCL.
Finally, we examined the associations between sleep duration as a continuous variable and behavior outcomes. To account for non-linear relations, we estimated adjusted mean differences with 95% CI in behavior scores between different sleep duration times and 8 hr, the reference value, using one linear and two nonlinear terms for sleep duration from restricted cubic spline models (Durrleman & Simon, Reference Durrleman and Simon1989). Because conventional sleep duration categories are age-specific and age could modify the associations of sleep duration with behavior outcomes, we tested for interactions between sleep duration and age. To identify the age at which associations between sleep duration and behavior differed, we computed χ2 statistics for interaction terms between a dichotomous variable representing a given age cut point and the 3 sleep duration terms in models for each outcome using likelihood ratio tests with 3 degrees of freedom. Statistics were computed for age cut points from 10 to 13 years, in 0.5 year increments. When an age cut point reached an interaction χ2 > 7.5, it was used to stratify the associations.
Analyses of the associations between adolescence sleep duration and behavior outcomes were conducted in a subset of 834 children (94%) who had information on this exposure (818 for YSR and 728 for CBCL). These analyses proceeded analogously to those for middle childhood sleep duration. Multivariable linear regression models with adolescence sleep duration were adjusted for adolescence sociodemographic and behavioral characteristics and for middle childhood sleep duration. Interactions between age and sleep duration were evaluated for age cut points from 14 to 17 years in 0.5 year increments. All analyses were performed using Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC).
Results
Middle childhood sleep duration and behavior problems in adolescence
Mean ± SD age at the time of sleep duration assessment was 10.4 ± 1.8 years; 56 % of children were girls. The proportion of children in sleep duration categories under, within, and above recommendations was 41%, 56%, and 3% overall; 43%, 55%, and 2% for those aged <13 years; and 16%, 66%, and 18% for those aged ≥13 years, respectively. Compared with children who slept within recommendations, those who slept under recommendations were taller and had higher vitamin B-12 serostatus and better educated mothers; whereas children who slept above recommendations were older and taller and had lower vitamin B-12 serostatus (Supplemental Table 2). These children also had mothers with higher parity and lower education and experienced more food insecurity. Mean ± SD age at the time of behavior assessment was 14.7 ± 1.7 years. The mean ± SD time elapsed between the middle childhood and adolescent assessments was 4.2 ± 1.1 years.
Externalizing problems
Compared with children who slept within recommendations, mean total externalizing scores per the YSR and the CBCL were, respectively, 4.6 (95% CI: 1.6, 7.6) and 5.4 (95% CI: 1.2, 9.7) units significantly higher in children who slept above recommendations (Table 1). These differences were mostly driven by associations with the aggressive behavior subscale (Table 1). Further adjustment for adolescence nighttime sleep duration (Table 1), nutritional characteristics, and a history of asthma or allergies (Supplemental Table 3) did not change these results. Although the associations seemed stronger in boys than girls, they did not differ significantly by sex for the YSR (Supplemental Table 4). There was no evidence that the associations between sleeping above recommendations and externalizing problems per the YSR were stronger for weekends than they were for weekdays (Supplemental Table 5). In analyses of sleep duration as a continuous exposure, the association with externalizing problems per the YSR differed between children <11 and ≥11 year-old at the time of sleep assessment (Supplemental Table 6). There was a positive, non-linear association between nighttime sleep duration and externalizing problems per the YSR, only in children aged ≥11 years (Figure 1). Compared with an 8 hr nighttime sleep, sleeping for 12 hr was related to an adjusted 3.6 (95% CI: 0.6, 6.5; p = .02) units higher total externalizing behavior score, whereas sleeping for 7 hr was related to an adjusted 2.1 (95% CI: −4.1, −0.1; p = .04) units lower score (Supplemental Table 7).
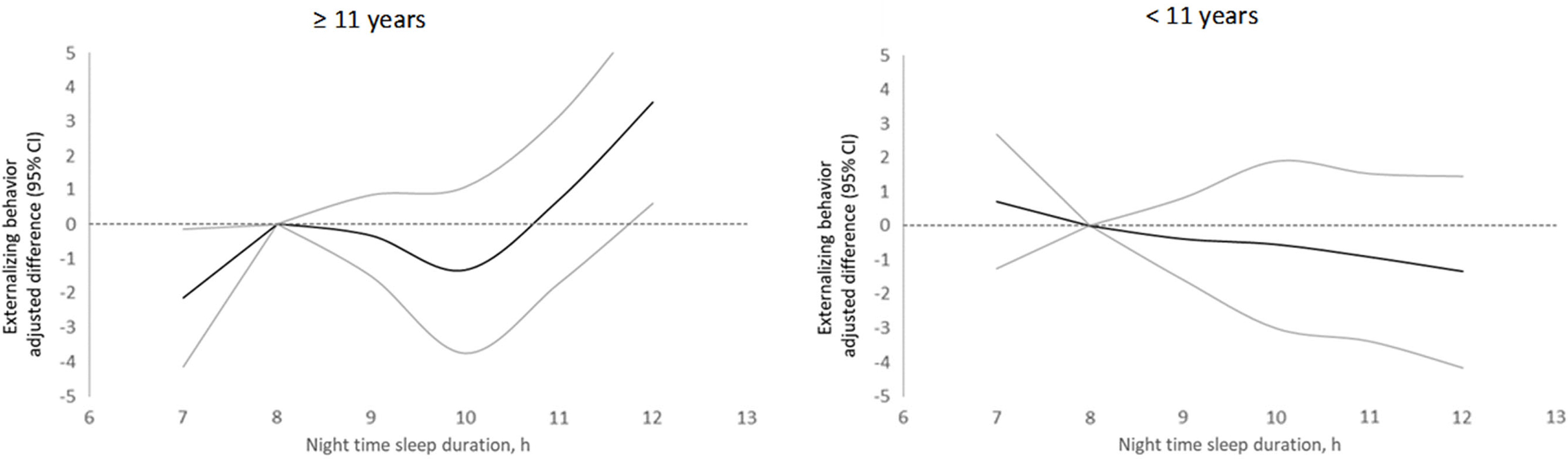
Figure 1 Adjusted differences in externalizing scores in adolescence per the YSR by nighttime sleep duration in middle childhood stratified by age at baseline. The dark line represents mean adjusted differences in the behavior score between a given sleep duration (hours) and 8 hours. Gray lines represent 95% confidence intervals. Estimates are from linear generalized estimating equation models with externalizing behavior score as the continuous outcome; predictors included linear and spline terms for sleep duration and sex, age, screen time, mother’s education, parity, and food insecurity. In all models, the robust sandwich estimate of the variance was used to account for intra-family correlations.
Table 1. Nighttime sleep duration in middle childhood and externalizing behavior problems in adolescence
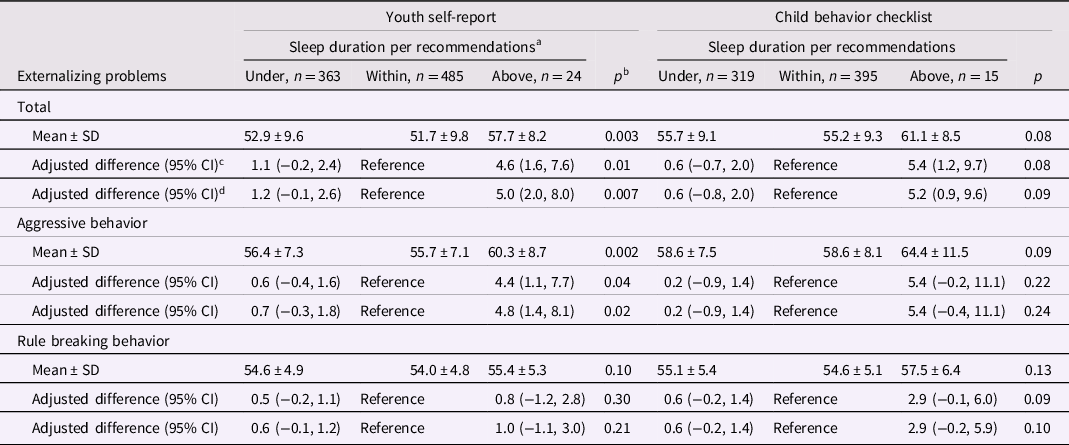
a <9, 9 to 12, or ≥12 hr per 24 hr, respectively, in children aged 6 to 12 years and <8, 8 to 10, or ≥10 hr per 24 hr in children aged ≥13 years.
b Kruskal–Wallis tests for unadjusted means and χ2 Score test for adjusted estimates.
c From linear generalized estimating equation models with each behavior problems score as the continuous outcome. Adjustment variables included child’s sex, age, screen time, mother’s education, parity, and food insecurity. Robust variances were specified in all models and accounted for within family correlations among children with siblings in the sample. Complete case analysis (n = 842 and 719 for YSR and CBCL models, respectively).
d Additionally adjusted for adolescence nighttime sleep duration in categories (under or above recommendations with within recommendations as reference). Complete case analysis (n = 799 and 718 for YSR and CBCL models, respectively).
Internalizing problems
Compared with sleeping within recommendations, sleeping above recommendations was associated with higher scores in total internalizing problems and their subscales per both YSR and CBCL (Table 2). Estimates were statistically significant for CBCL total internalizing, anxious/depressed, and somatic complaints scores, and for YSR somatic complaints. Further adjustment for adolescence nighttime sleep duration (Table 2), nutritional factors, asthma, or allergies (Supplemental Table 8) did not substantially change the associations. Sex did not significantly modify the associations between sleeping above recommendations and internalizing behavior per the YSR (Supplemental Table 9). The associations with total internalizing, anxious/depressed, and somatic complaints scores per the YSR seemed stronger for weekday than for weekend sleep above recommendations (Supplemental Table 10). In analysis of sleep duration as a continuous variable, the association with internalizing problems per the CBCL did not differ by age (Supplemental Table 11). Compared with an 8 hr nighttime sleep, sleeping for 12 hr was related to an adjusted 3.9 (95% CI: 1.7, 6.1; p = .0004) units higher total internalizing behavior score (Supplemental Table 12, Figure 2).
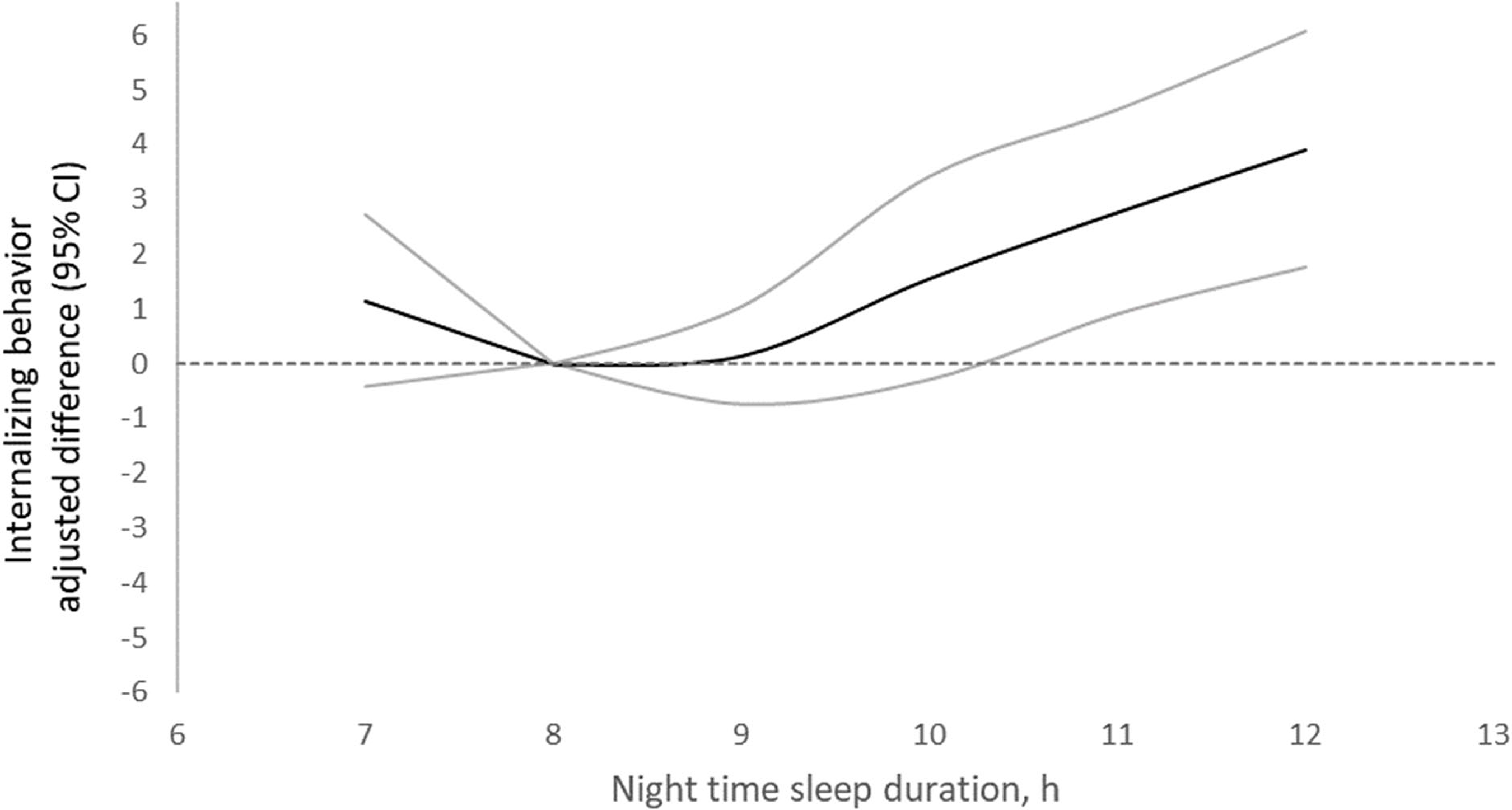
Figure 2 Adjusted differences in internalizing scores in adolescence per the CBCL by nighttime sleep duration in middle childhood stratified by age at baseline. The dark line represents mean adjusted differences in the behavior score between a given sleep duration (hours) and 8 hours. Gray lines represent 95% confidence intervals. Estimates are from linear generalized estimating equation models with internalizing behavior score as the continuous outcome; predictors included linear and spline terms for sleep duration and sex, age, screen time, mother’s education, parity, and food insecurity. In all models, the robust sandwich estimate of the variance was used to account for intra-family correlations.
Table 2. Nighttime sleep duration in middle childhood and internalizing behavior problems in adolescence
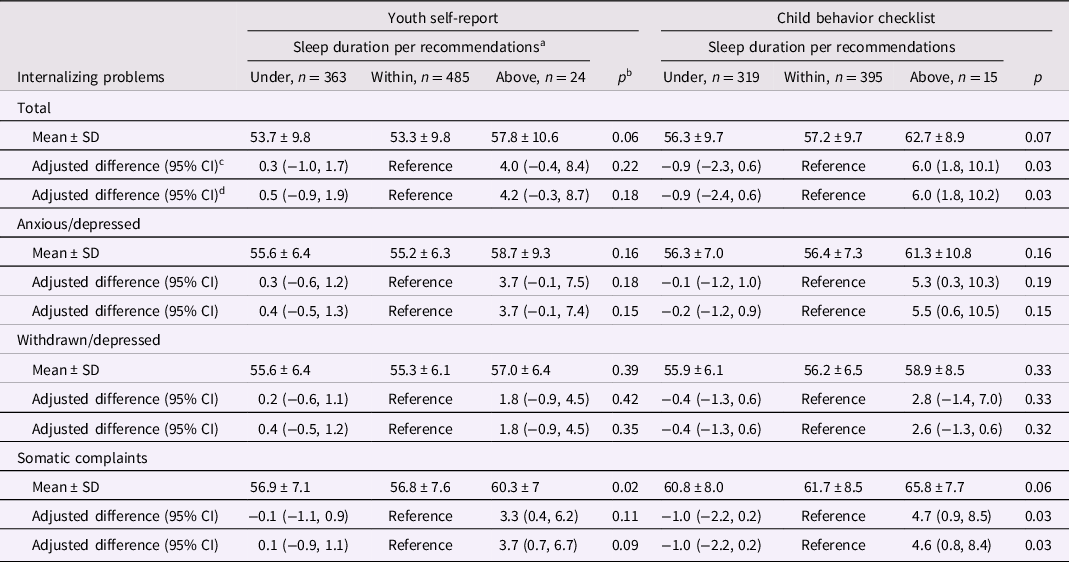
a <9, 9 to 12, or ≥12 hr per 24 hr, respectively, in children aged 6 to 12 years and <8, 8 to 10, or ≥10 hr per 24 hr in children aged ≥13 years.
b Kruskal–Wallis tests for unadjusted means and χ2 Score test for adjusted estimates.
c From linear generalized estimating equation models with each behavior problems score as the continuous outcome. Adjustment variables included child’s sex, age, screen time, mother’s education, parity, and food insecurity. Robust variances were specified in all models and accounted for within family correlations among children with siblings in the sample. Complete case analysis (n = 842 and 719 for YSR and CBCL models, respectively).
d Additionally adjusted for adolescence nighttime sleep duration in categories (under or above recommendations with within recommendations as reference). Complete case analysis (n = 799 and 718 for YSR and CBCL models, respectively).
Attention, social, and thought problems
Sleeping above recommendations was related to an adjusted 4.5 (95% CI: 1.7, 7.4) units higher social problems per the YSR than sleeping within recommendations (Table 3). Further adjustment for adolescence nighttime sleep duration (Table 3), nutritional factors, asthma, and allergies (Supplemental Table 13) did not change this association. Sex did not significantly modify the associations of sleep duration with any of these scales per the YSR (Supplemental Table 14). The relation of sleeping above recommendations with social problems per the YSR seemed stronger for sleeping above recommendations during weekdays than during weekends (Supplemental Table 15). Sleep duration as a continuous variable was not significantly related to social problems per the YSR (Supplemental Tables 6 and 7) or the CBCL (Supplemental Tables 11 and 12).
Table 3. Nighttime sleep duration in middle childhood and attention, social, and thought problems in adolescence
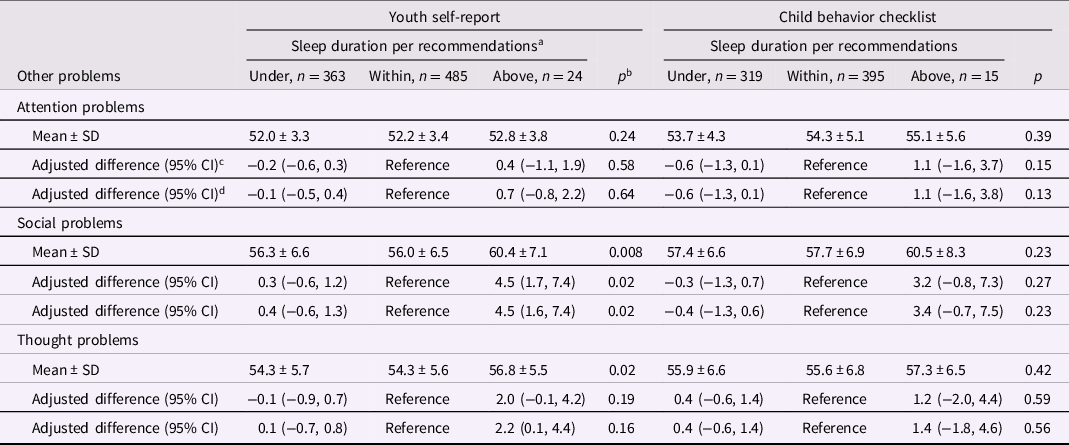
a <9, 9 to 12, or ≥12 hr per 24 hr, respectively, in children aged 6 to 12 y and <8, 8 to 10, or ≥10 h per 24 h in children aged ≥13 years.
b Kruskal–Wallis tests for unadjusted means and χ2 Score test for adjusted estimates.
c From linear generalized estimating equation models with each behavior problems score as the continuous outcome. Adjustment variables included child’s sex, age, screen time, mother’s education, parity, and food insecurity. Robust variances were specified in all models and accounted for within family correlations among children with siblings in the sample. Complete case analysis (n = 842 and 719 for YSR and CBCL models, respectively).
d Additionally adjusted for adolescence nighttime sleep duration in categories (under or above recommendations with within recommendations as reference). Complete case analysis (n = 799 and 718 for YSR and CBCL models, respectively).
Adolescence sleep duration and behavior problems in adolescence
The proportion of children in sleep duration categories under, within, and above recommendations was 39%, 50%, and 11%, respectively. Adolescence sleep duration was positively related to middle childhood sleep duration and maternal parity and inversely related to BMI-for-age, maternal education, and household SES (Supplemental Table 16).
Externalizing problems
Compared with children who slept within recommendations, mean total externalizing scores per the YSR were 1.8 (95% CI: 0.5, 3.2) units significantly higher in children who slept under recommendations (Table 4). These differences were mostly driven by associations with the aggressive behavior subscale (Table 4). In analyses of sleep duration as a continuous exposure, shorter sleep duration was associated with externalizing problems per the YSR only in children <15 year-old (Supplemental Table 17). Compared with an 8 hr nighttime sleep, sleeping for 7 hr was related to an adjusted 1.8 (95% CI: 0.2, 3.6; p = .03) units higher total externalizing behavior score per the YSR (Supplemental Table 18, Supplemental Figure 1).
Table 4. Nighttime sleep duration in adolescence and externalizing behavior problems in adolescence
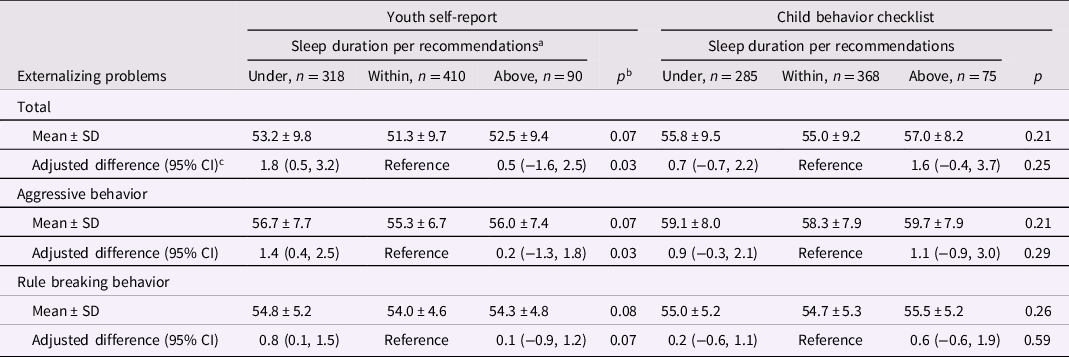
a < 9, 9 to 12, or ≥12 hr per 24 hr, respectively, in children aged 6 to 12 years and <8, 8 to 10, or ≥10 h per 24 h in children aged ≥13 years.
b Kruskal–Wallis tests for unadjusted means and χ2 Score test for adjusted estimates.
c From linear generalized estimating equation models with each behavior problems score as the continuous outcome. Adjustment variables included child’s age, body mass index-for-age Z score, and socioeconomic status at the adolescence assessment, and sex and middle childhood sleep duration. Robust variances were specified in all models and accounted for within family correlations among children with siblings in the sample. Complete case analysis (n = 818 and 728 for YSR and CBCL models, respectively).
Internalizing, attention, social, and thought problems
There were no significant associations between sleeping above or under recommendations and internalizing (Table 5), attention, social, or thought problems (Table 6). In analyses of adolescence sleep duration as a continuous variable, scores for YSR total internalizing problems, social problems in children <15 year-old (Supplemental Table 17), and thought problems were significantly higher in children sleeping for 7 hr compared with an 8 hr nighttime sleep (Supplemental Table 18). In children ≥17 year-old, sleeping for 12 hr was associated with significantly higher CBCL total internalizing scores sleeping compared to sleeping for 8 hr (Supplemental Tables 19 and 20).
Table 5. Nighttime sleep duration in adolescence and internalizing behavior problems in adolescence
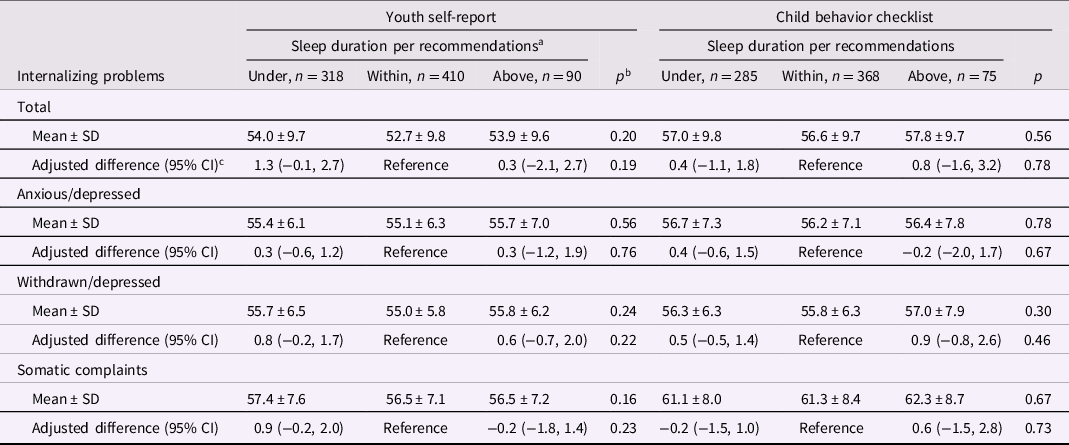
a <9, 9 to 12, or ≥12 h per 24 h, respectively, in children aged 6 to 12 years and <8, 8 to 10, or ≥10 h per 24 h in children aged ≥13 years.
b Kruskal–Wallis tests for unadjusted means and χ2 Score test for adjusted estimates.
c From linear generalized estimating equation models with each behavior problems score as the continuous outcome. Adjustment variables included child’s age, body mass index-for-age Z score, and socioeconomic status at the adolescence assessment, and sex and middle childhood sleep duration. Robust variances were specified in all models and accounted for within family correlations among children with siblings in the sample. Complete case analysis (n = 818 and 728 for YSR and CBCL models, respectively).
Table 6. Nighttime sleep duration in adolescence and attention, social, and thought problems in adolescence
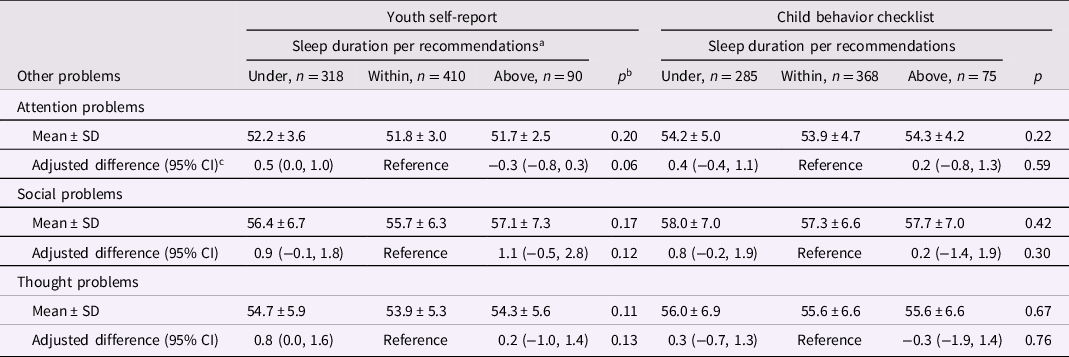
a <9, 9 to 12, or ≥12 hr per 24 hr, respectively, in children aged 6 to 12 y and <8, 8 to 10, or ≥10 hr per 24 hr in children aged ≥13 years.
b Kruskal–Wallis tests for unadjusted means and χ2 Score test for adjusted estimates.
c From linear generalized estimating equation models with each behavior problems score as the continuous outcome. Adjustment variables included child’s child’s age, body mass index-for-age Z, and socioeconomic status at the adolescence assessment, and sex and middle childhood sleep duration. Robust variances were specified in all models and accounted for within family correlations among children with siblings in the sample. Complete case analysis (n = 818 and 728 for YSR and CBCL models, respectively).
Discussion
In this longitudinal study of Colombian school children, sleeping above recommendations in middle childhood was associated with externalizing and internalizing behavior problems in adolescence. The association with externalizing problems was driven primarily by aggressive behavior with no discernable differences between weekends and weekdays. Among internalizing problems, anxious/depressed behavior and somatic complaints scores increased with sleeping above recommendations; these associations were slightly stronger on weekdays compared to weekends. Results from analyses of middle childhood sleep duration as a continuous exposure were generally consistent with categorical analyses; although, the association with externalizing problems seemed restricted to children ≥11 years at the time of exposure assessment. In addition, sleeping above recommendations in middle childhood was associated with higher social problem scores in adolescence. In contrast with the results for middle childhood sleep duration, shorter sleep duration in adolescence was associated with externalizing problems in both the categorical and the continuous exposure analyses. Shorter adolescence sleep duration was also related to YSR total internalizing behavior and social and thought problems in continuous exposure analyses. Associations were independent of child, maternal, and household factors; also, associations with middle childhood sleep duration were independent of those with adolescence sleep duration and vice versa.
Few studies have reported on the role of sleep duration above recommendations and externalizing behavior in children. A longitudinal investigation of 1,965 children in the Fragile Families and Child Wellbeing Study (FFCWS) found a positive association between sleep duration above recommendations at age 9 years and externalizing behavior problems later in life (James & Hale, Reference James and Hale2017a), consistent with our findings for middle childhood sleep. The same and other studies also noted an association between short sleep duration in childhood and externalizing problems (Gregory et al., Reference Gregory, van der Ende, Willis and Verhulst2008; Nixon et al., Reference Nixon, Thompson, Han, Becroft, Clark, Robinson, Waldie, Wild, Black and Mitchell2008; Ranum et al., Reference Ranum, Wichstrøm, Pallesen, Falch-Madsen, Halse and Steinsbekk2019; Scharf et al., Reference Scharf, Demmer, Silver and Stein2013; Weaver et al., Reference Weaver, Barger, Malone, Anderson and Klerman2018). While we similarly found associations between short sleep duration in middle childhood and externalizing behavior, they were weaker and lacked statistical significance compared with those for sleep above recommendations. Our findings highlight the importance of examining sleep above, within, and under recommendations as separate exposure categories.
In our study, long sleep duration in middle childhood was related to internalizing problems in adolescence. This is consistent with results from the FFCWS (James & Hale, Reference James and Hale2017b) and from a longitudinal investigation of Chinese adolescents in which long sleep duration on weekdays and weekends was positively associated with depressive symptoms after 2 years (Liu et al., Reference Liu, Wang, Liu, Wang, An, Wei, Jia and Liu2020). Long sleep duration in children is more common on weekends than it is on weekdays and can result from recovery or catchup sleep (Gradisar et al., Reference Gradisar, Gardner and Dohnt2011). Thus, long sleep duration on weekdays may be a better measure of consistent oversleeping than long sleep on weekends. We found a stronger association between sleeping above recommendations on weekdays than on weekends and internalizing problems; which suggests a potential role of habitual long sleep duration on behavior development. Further research is warranted to clarify the association of variability between weekday and weekend sleep timing and duration with behavior problems.
Different mechanisms could explain an association between long sleep duration and the development of behavior problems. Nightmares could be one of them; sleeping ≥9 hr is associated with nightmares (Munezawa et al., Reference Munezawa, Kaneita, Osaki, Kanda, Ohtsu, Suzuki, Minowa, Suzuki, Higuchi, Mori and Ohida2011; Sandman et al., Reference Sandman, Valli, Kronholm, Revonsuo, Laatikainen and Paunio2015) which, in turn, are related to both internalizing and externalizing problems (Li et al., Reference Li, Yu, Lam, Zhang, Li, Lai and Wing2011). Medical conditions such as asthma and allergies may be associated with sleeping above recommendations (Koinis-Mitchell et al., Reference Koinis-Mitchell, Craig, Esteban and Klein2012). However, adjustment for these factors did not change the association between sleep duration and behavior. Furthermore, sleep duration is associated with lower quality of life and decreased social interactions which can contribute to internalizing behavior (Ohayon et al., Reference Ohayon, Reynolds and Dauvilliers2013).
We found a consistent contrast in the direction of the associations between behavior problems and sleep duration in middle childhood vs. adolescence. While behavior problems were related to long sleep duration in middle childhood, they were generally associated with short sleep in adolescence. The association of short sleep duration and behavior problems in adolescence is well documented. Meta-analyses of epidemiologic studies suggest a consistent inverse association between sleep duration and externalizing and internalizing problems in adolescence (Lovato & Gradisar, Reference Lovato and Gradisar2014; van Veen et al., Reference van Veen, Lancel, Beijer, Remmelzwaal and Rutters2021). Also, experimental investigations involving sleep restriction have shown the deleterious effects of short sleep duration on mood and cognitive processing (Baum et al., Reference Baum, Desai, Field, Miller, Rausch and Beebe2014; Lo et al., Reference Lo, Ong, Leong, Gooley and Chee2016). Different mechanisms could explain the impact of short sleep duration on behavior. Short sleep duration is associated with poor memory consolidation and cognitive performance which could lead to aggressive behavior through impaired school performance (Alhola & Polo-Kantola, Reference Alhola and Polo-Kantola2007; Vuoksimaa et al., Reference Vuoksimaa, Rose, Pulkkinen, Palviainen, Rimfeld, Lundström, Bartels, van Beijsterveldt, Hendriks, de Zeeuw, Plomin, Lichtenstein, Boomsma and Kaprio2021). In addition, short sleep duration has a demonstrated effect on the activation of the amygdala when children are exposed to negative emotional stimuli (Yoo et al., Reference Yoo, Gujar, Hu, Jolesz and Walker2007). Activation of the amygdala is related to inhibited behavior regulation and emotional instability (Davidson, Reference Davidson2002). In exception to these adolescence findings, long sleep duration was associated with CBCL total internalizing scores in children ≥17 year-old. This singular association between long sleep duration and internalizing problems in adolescence may reflect reverse causation bias because oversleeping is a common symptom of internalizing behaviors such as depression (Nutt et al., Reference Nutt, Wilson and Paterson2008).
The contrast in the direction of the associations of behavior problems with sleep duration in middle childhood vs. adolescence in the same population is a novel finding yet challenging to explain. It is possible that the middle childhood assessment represents cumulative or long-term effects whereas the cross-sectional results in adolescence indicate an acute effect of current sleep duration. Alternatively, the cross-sectional analyses in adolescence may be more prone to reverse causation bias (i.e., behavior problems causing short sleep duration) than the longitudinal study of middle childhood sleep duration. Also, because adolescence sleep duration was self-reported at the same time as the behavior assessment, measurement error correlations between exposure and outcome may be larger than those of behavior with sleep duration in middle childhood, which was reported by parents several years before outcome assessment. A high exposure-outcome measurement error correlation could introduce bias.
There are several strengths of this study. First, its longitudinal design reduces the possibility of reverse causation in the associations of middle childhood sleep duration and adolescence behavior. Differential misclassification of exposure is unlikely due to the prospective nature of outcome data collection. Second, we had an opportunity to examine the associations separately for weekdays and weekends sleep duration; this is advantageous because weekday sleep duration may better represent habitual sleep than that over weekends. Third, outcomes were assessed with the use of two complementary instruments involving both self- and maternal report. The consistency of the findings between the two outcome assessment methods indicates high internal validity. Fourth, the modeling strategy reduced confounding for many child, maternal, and household factors.
Some limitations are also worth noting. First, reverse causation cannot be completely ruled out as an explanation of the findings. Establishing the temporal directionality of the relation between long sleep duration in middle childhood and behavior problems can be challenging in our study because we lacked behavior information at the time of sleep duration assessment in middle childhood. If behavior problems were already present in middle childhood, they could cause sleep duration rather than the opposite; for example, internalizing behaviors involving depression could cause sleep problems (Nutt et al., Reference Nutt, Wilson and Paterson2008). Second, exposure sleep data was limited to information about sleep duration and did not include measures of sleep quality and other relevant sleep problems like apnea, insomnia, nightmares, and excessive daytime sleepiness (Moturi & Avis, Reference Moturi and Avis2010). Insufficient or excessive sleep could also be symptoms of some of these sleep disorders including apnea, and nightmares (Owens, Reference Owens2008). Third, although we adjusted the associations for many factors related to sleep and behavior, the study lacked information on middle childhood potential confounders like bedtime, pubertal development status, circadian rhythm, and child morningness vs eveningness (Kroese et al., Reference Kroese, Evers, Adriaanse and de Ridder2016; McGlinchey & Harvey, Reference McGlinchey and Harvey2015; Murray et al., Reference Murray, Phillips, Magee, Sletten, Gordon, Lovato, Bei, Bartlett, Kennaway, Lack, Grunstein, Lockley, Rajaratnam, Armstrong, Chohan, Djavadkhani, Dodds, Gunaratnam, Hardy and Yu2019; Pieters et al., Reference Pieters, Vorst, Burk, Wiers and Engels2010). Thus, residual confounding cannot be ruled out. Fourth, sleep data exposure was collected through parental report in middle childhood and through self-report in adolescence. The questions had not been validated in this population. Parental or self-report of sleep are not as accurate as objective sleep assessment methods such as polysomnography. It was not possible to ascertain the duration of sleep stages and cycles or their relations with behavior outcomes. Fifth, the questions about sleep duration do not specify an exposure period. Thus, responses may be influenced by recency bias and misrepresent the overall sleeping habits. Sixth, approximately 5% of the children were already adolescents at the time of sleep exposure assessment in middle childhood. Finally, the results of this study may not be widely generalizable to populations with different characteristics.
In conclusion, sleeping above recommendations in middle childhood is related to externalizing, internalizing, and social problems in adolescence. By contrast, short sleep duration in adolescence is associated with behavior problems. Additional studies are warranted to elucidate the role of changes in sleep characteristics during childhood and adolescence with the development of behavior problems and other health outcomes.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579422001237
Funding statement
This work was supported by the ASISA Foundation.
Conflicts of interest
None.













