Introduction
Giardia (Metamonada, Giardiidae) is an enteric flagellated diplomonad protozoan parasite that infects a wide range of mammalian hosts including humans and animals, leading to one of the most frequently occurring parasitic diseases known as giardiasis (Xu et al., Reference Xu, Jiménez-González, Einarsson, Ástvaldsson, Peirasmaki, Eckmann, Andersson, Svärd and Jerlström-Hultqvist2020). The infection can be asymptomatic in some cases, and when symptoms do appear, they can range from persistent diarrhoea, abdominal pain, by severe malabsorption, all of which can have a negative influence on growth and intellectual development (Ramírez et al., Reference Ramírez, Heredia, Hernández, León, Moncada, Reyes, Pinilla and Lopez2015). This protist is ubiquitously distributed and is responsible for cases of human diarrhoea annually, mostly in children <5 years of age with lower prevalence in developed compared to developing countries (Feng and Xiao, Reference Feng and Xiao2011; Mahdavi et al., Reference Mahdavi, Sadrebazzaz, Chahardehi, Badali, Omidian, Hassanipour and Asghari2022) and can infect over 40 animal species (Thompson and Monis, Reference Thompson and Monis2004; Taghipour et al., Reference Taghipour, Sharbatkhori, Tohidi, Ghanbari, Karanis, Olfatifar, Majidiani, Khazaei, Bahadory and Javanmard2022). Life cycle of G. duodenalis begins when the infective cyst forms are shed into the environment in fecal material; excystation occurs which is enhanced by the gastric acid and pancreatic enzymes after ingestion by another host forming 2 motile pear-shaped trophozoites that subsequently colonize the small intestine (duodenum and jejunum) provoking conjugation and lipid metabolism dysfunction (Li et al., Reference Li, Wang, Wang and Zhang2017; Buret et al., Reference Buret, Cacciò, Favennec and Svärd2020).
Ingestion of cysts from polluted water or food causes infection in humans and other mammals (House et al., Reference House, Richter, Pham and Dawson2011). Transmission of this parasite is direct via the fecal–oral route, as in the case of farmers, veterinarians and petting zoos, or indirectly, as in polluted surface water or foods (Hunter and Thompson, Reference Hunter and Thompson2005; Dixon et al., Reference Dixon, Parrington, Cook, Pintar, Pollari, Kelton and Farber2011). Water sources infected with cysts from fecal deposition or sewage disposal techniques are the most common sources of Giardia infection in humans (Solarczyk et al., Reference Solarczyk, Dabert, Frantz, Osten-Sacken, Trzebny, Wojtkowiak-Giera and Heddergott2021). Dogs and cats that are kept as pets could also serve as major zoonotic transmission route to humans (Aw et al., Reference Aw, Clarke, McCarthy, Traub, Amaral, Huque, Andrews, Gray, Clements and Vaz Nery2019).
Globally, the detection, identification and characterization of Giardia are central to investigating and understanding the epidemiology of giardiasis. Diagnostic methods that have been employed broadly include fecal microscopy, immunodiagnostics and molecular techniques (Hooshyar et al., Reference Hooshyar, Rostamkhani, Arbabi and Delavari2019).
Giardiasis has been included in the Neglected Disease Initiatives of the World Health Organization (WHO) since September 2004 due to its health effects on children and pregnant women as well as being associated with poverty (Mirrezaie et al., Reference Mirrezaie, Beiromvand, Tavalla, Teimoori and Mirzavand2019). The incidence of Giardia in the resource-poor countries is estimated to be 20–30% due to hazardous water supplies, sanitation and hygiene (Groudan et al., Reference Groudan, Gupta, Chalhoub and Singhania2021). In sub-Saharan Africa and West Africa, prevalences are estimated to be 7.36 and 8.97%, respectively (Bogoch et al., Reference Bogoch, Raso, N'Goran, Marti and Utzinger2006; Belhassen-García et al., Reference Belhassen-García, Pardo-Lledías, Del Villar, Velasco-Tirado, Ruiz, Cordero-Sánchez, Vicente, Egido, Bellido and Muro2017). We performed a systematic review and meta-analysis using a ‘One Health’ approach in order to better define the prevalence and epidemiological distribution of Giardia species in animals, humans and waterbodies from published literature between 1980 and 2022 in the African continent. We are of the opinion that the outcomes of this study will be useful to policy makers on how to minimize the burden of giardiasis in developing countries.
Materials and methods
Protocol and registration
The protocol for this study was preregistered in PROSPERO with registration number CRD42022317653. We performed a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan and Chou2021) which have been confirmed on a checklist (Supplementary Table S1).
Search strategy
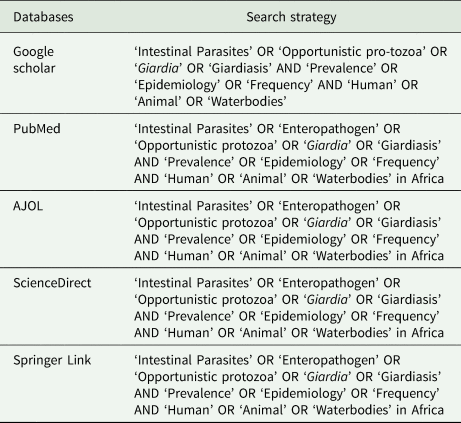
Literature searches were conducted using keywords (Table 1) on PubMed, ScienceDirect, AJOL, SpringerLink and Google Scholar on articles published in English language from 1 January 1980 until 22 March 2022 for articles with emphasis on the prevalence or epidemiology of Giardia species across the continent of Africa in animals, humans and waterbodies. None of the authors of original studies were contacted for additional information and no attempt was made to retrieve unpublished articles. Titles and abstracts were scanned, and relevant full-text articles were downloaded and obtained through library resources and online platforms.
Table 1. Search strategy
Eligibility criteria
Inclusion and exclusion criteria
Articles were included only if they fulfilled the following inclusion criteria: cross-sectional (prevalence) study conducted within African continent; study involving the detection and/or screening of vertebrate hosts (humans or animals), waterbodies including fecal and water samples for Giardia duodenalis (sny. G. intestinalis, G. lamblia); the exact total numbers and positive cases were clearly provided; sample size (>50 to enable statistical computations); published study written in English language; study conducted between 1 January 1980 until 22 March 2022. Studies were excluded if (i) they were conducted outside of Africa, (ii) case control or randomized studies, (iii) involved the detection of Giardia in fresh produce or soil, (iv) incomplete data on the total number of samples screened or number of positives obtained, (v) published papers outside the study periods and (vi) written in other languages.
Study selection and data extraction
Independent reviewers (M. T. and T. O.) carefully evaluated all titles and abstracts identified in the search, as well as full texts considered to be relevant. Any disagreements were resolved by discussion with the other 2 authors (T. R. and O. T.). Titles and abstracts derived through primary electronic search were thoroughly assessed for possibility of inclusion based on the study type (prevalence of Giardia in animals, humans and waterbodies), and duplicates were removed. Full texts were examined and unrelated studies were excluded with reasons. All studies that met the eligibility criteria were included for syntheses. From each eligible study, the following data were extracted and organized using Microsoft Excel spreadsheet using the format: name of the author and countries, study/publication year, country, hosts, total sample size, number of positive cases, estimated prevalence, consistency of the feces and different diagnostic technique. Studies that were conducted in more than 1 country and those that had both animal, human and waterbodies studies simultaneously were separated accordingly.
Quality assessment of included studies/risk of bias
The risk of bias for each study was assessed using the Joanna Briggs Institute (JBI) Critical Appraisal Tools for cross-sectional study (Munn et al., Reference Munn, Moola, Lisy, Riitano and Tufanaru2015). This JBI instrument consists of 9 criteria, of which details are available (Supplementary Table S2). Each response to the individual criteria was assigned a score of 0 or 1 for no or yes answers. When the question was not applicable to the study, not applicable (NA) was used. A maximum score of 9 was possible but only 8 was applicable to our kind of study that was eligible for incorporation in this review. Studies with scores of 7–8 indicated a low risk of bias, scores of 5–6 indicated a moderate risk of bias, and scores less than 5 indicated a high risk of bias.
Data synthesis
The current meta-analysis was conducted using the Comprehensive Meta-Analysis software (CMA) version 3.0 software (Borenstein et al., Reference Borenstein, Hedges, Higgins and Rothstein2014). The pooled prevalence estimates (PPE) and 95% confidence interval (CI) were calculated using random-effects models. Statistical heterogeneity between studies was measured by I 2 statistic (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). Publication bias was measured using funnel plots to test for symmetry and this was further complimented using the Begg's and Mazumdar rank (BMR) correlation test (Begg and Mazumdar, Reference Begg and Mazumdar1994).
Results
Search results
A total of 6222 studies were retrieved following the initial search from 5 databases (Fig. 1). A total of 2242 articles were removed as they were duplicates and the remaining articles (n = 3980) were screened based on titles and abstracts. Thereafter, a total of 3270 was removed as unlikely leaving 710 studies that were subjected for eligibility and were thus examined by full-text evaluation. Exactly 206 articles were excluded with reasons as follows: studies conducted outside of Africa (n = 97), studies with no clarity on data (n = 66), studies with focus on animal experiment (n = 21) and lastly, studies with different types of samples (n = 22). Ultimately, for the quantitative synthesis (meta-analysis) of eligible studies, a total of 426, 67 and 24 studies were used for human, animal and waterbodies, respectively, to obtain the PPE (Fig. 1).
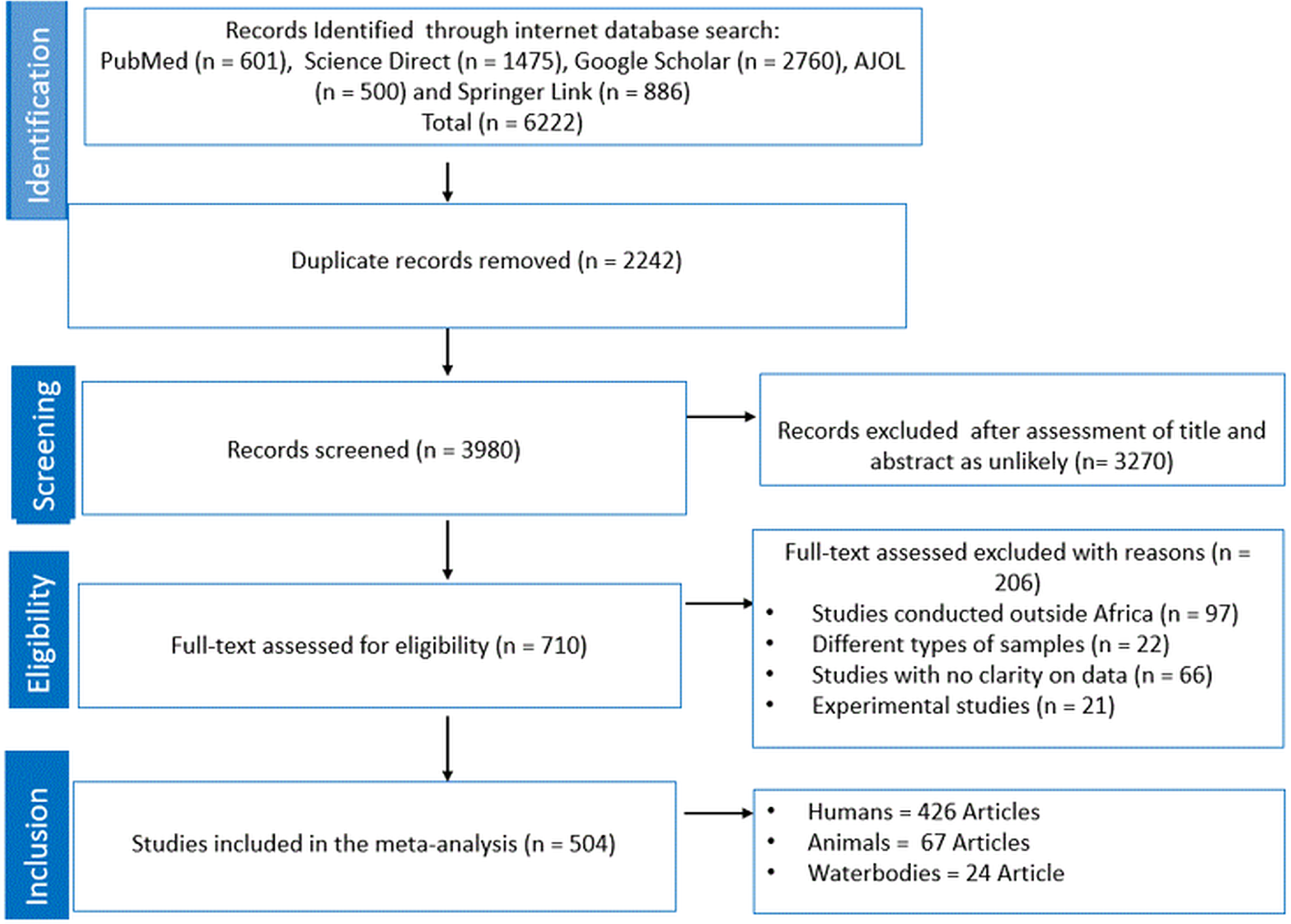
Figure 1. PRISMA flow chart.
General characteristics of the included studies
The characteristics of all eligible studies included in this review are presented in Supplementary Tables S3–S5. All studies were published from 1980 to 2022, with the majority of the studies conducted in the last 2 decades (2002–2022). The prevalence for all the individual studies was computed and presented in Supplementary Table S1. All studies were conducted in different parts of Africa, with eligible studies with focus on humans widely distributed across the continent in different countries. Exactly 34 countries documented the existence of Giardia species in samples collected from humans with Ethiopia (n = 115) having the highest number of studies, followed by Nigeria and Egypt with 53 studies each. Others include Kenya (n = 31), Ghana (n = 26), Guinea (n = 15) and South Africa (n = 14). Details of the findings from other countries are presented in Fig. 1. For studies pertaining to animals, Uganda (n = 11) and Nigeria (n = 9) had high number of eligible studies. Lastly, both Egypt and South Africa had 6 published eligible studies with interest on Giardia species from samples collected from waterbodies. Diagnostic technique employed across the 3 (human, animals and waterbodies) different subjects of interest includes microscopy, copro-antigen tests and molecular-based diagnostics.
Quality assessment of included studies
The quality assessment score of included studies ranged from 7 to 8 as per JBL critical appraisal checklist for studies reporting Giardia spp. prevalence data. About 10, 6 and 11 studies were excluded from this systematic review and meta-analysis for humans, animals and waterbodies, respectively, since they scored less than 66.7%.
PPE of G. duodenalis infection in humans
Different diagnostic methods were utilized for the detection of Giardia spp. infections from stool samples collected from humans across the continent. Of the 494 014 stool samples examined for Giardia spp. infection, 48 124 cases were registered as positives using microscopy. Thus, the PPE was 8.8% (95% CI 8.0–9.6%) (Table 2). Using copro-antigen tests, the PPE of anti-Giardia spp. was 14.3% (95% CI 10.1–20.0%). Lastly, a total of 16 095 stool samples were examined for the prevalence of Giardia spp. infection using molecular-based methods, out of which 2437 samples were positive with PPE of 19.5% (95% CI 13.0–24.3%) (Table 2). According to gender, the PPE in males was 13.3% (95% CI 11.7–15.1%) compared to 11.9% (95% CI 10.3–13.8%) in females. Furthermore, G. duodenalis infections were more prevalent in human subjects within the age range 0–18 years with PPE of 13.4% (95% CI 10.9–16.4%) while those within 19–35 years had the least PPE of 8.8% (95% CI 7.1–10.8%). The PPE in human subjects in rural areas was 17.9% (95% CI 13.0–24.3%) comparatively higher to human subjects in urban areas 9.2% (95% CI 6.3–13.1%) (Table 2). Additionally, the PPE of giardiasis was notably observed in the 1981–1990 year interval at 11.4% (95% CI 7.5–17.0%), followed by 9.6% (95% CI 7.4–12.3%) in 2001–2010, 9.6% (95% CI 8.6–10.6%) in 2011–2022 and 7.9% (95% CI 5.4–11.6%) in 1990−2000 year interval (Table 2). Human subjects that were HIV+ had a higher PPE of giardiasis at 5.0% (95% CI 3.5–7.3%) compared to those who were HIV– at 4.1% (95% CI 2.5–6.6%). Based on stool consistency, patients with diarrhoetic stool had a higher PPE (12.3%; 10.3–14.7%) compared to non-diarrhoetic patients (9.7%; 7.2–12.9%). Finally, the PPEs at country level indicate that Tunisia registered the highest at 39.9% (with only 2 eligible studies) while the lowest PPE was registered in Cameroon 1.3% (95% CI 0.4–4.1%) (Table 2).
Table 2. Sub-group analysis of Giardia duodenalis infection in human subjects across Africa

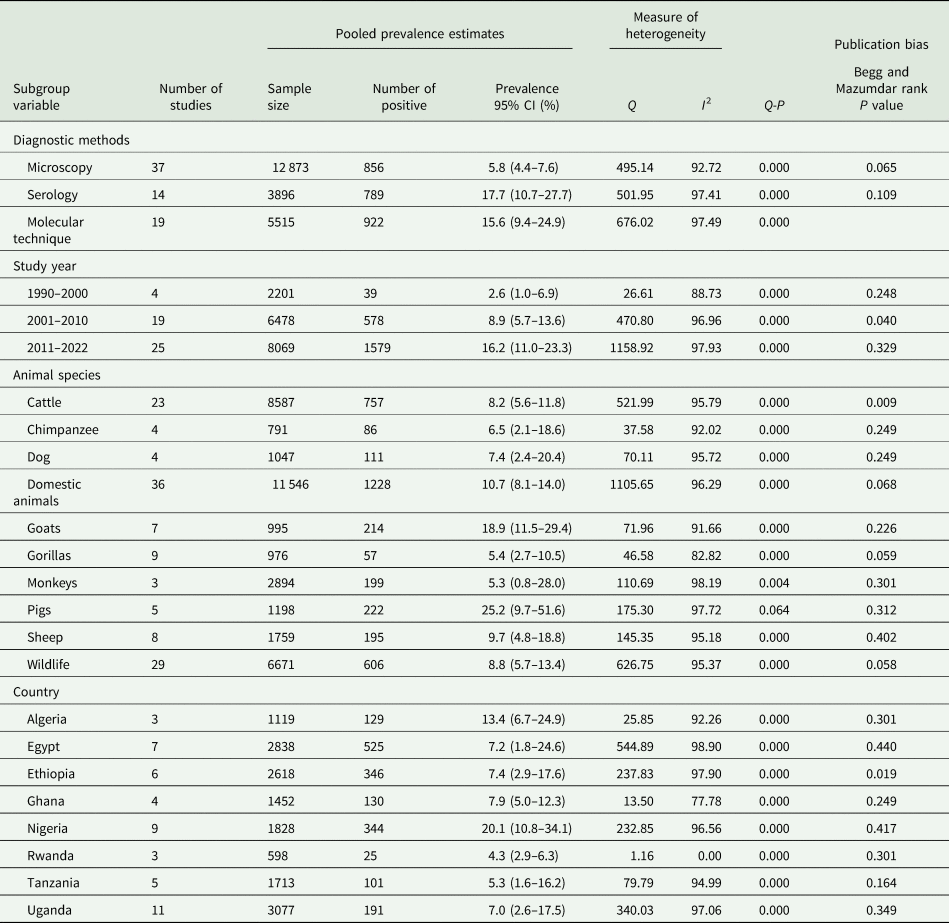
PPE of G. duodenalis infection in animals
Just like in human subjects, 3 diagnostic techniques were utilized in the detection of Giardia spp. infection in humans. Of the 12 873 fecal samples screened from animals using microscopy, a total of 856 were positive with PPE at 5.8% (95% CI 4.4–7.6%) (Table 3). Using serology, 789 samples were positive to anti-Giardia spp. out of a total of 3896 samples screened with an estimated PP at 17.7% (95% CI 10.7–27.7%) (Table 3). From a total of 5515 samples, 922 were positive for Giardia spp. infections using molecular methods with a PPE of 15.6% (95% CI 9.4–24.9%) (Table 3). According to study year, Giardia spp. infections were more prevalent in the 2011–2022 year interval with PPE of 16.2% (95% CI 11.0–23.3%), followed by 8.9% (95% CI 5.7–13.6) in 2001–2010, and then 2.6% (95% CI 1.0–6.9%) in 1990–2000 year interval (Table 3). Based on animal host, pigs had the highest PPE of 25.2% (95% CI 9.7–51.6%) followed by goats of 18.9% (95% CI 11.5–29.4) and the lowest was observed in monkeys of 5.3% (95% CI 0.8–28.0%) (Table 3). The PPE according to country level indicates that Nigeria had the highest at 20.1% (95% CI 10.8–34.1%) and Rwanda with the lowest at 4.3% (95% CI 2.9–6.3%) (Table 3).
Table 3. Sub-group analysis of Giardia species infection in animals' species across Africa
PPE of G. duodenalis contamination in waterbodies
A total of 7950 samples from various waterbodies across the continent were examined for the prevalence of Giardia spp. contamination, out of which 1407 water samples were positive using microscopy with PPE of 11.9% (95% CI 7.7–18.0%) (Table 4). Furthermore, of the 1288 water samples examined for the prevalence of Giardia spp. contamination, a total of 454 samples were positive using molecular methods, with PPE of 32.0% (95% CI 24.7–40.2%). The Giardia parasite was most prevalent in the 2011–2022 year interval, with PPE of 25.3% (95% CI 13.8–41.6%) as compared to 2001–2010 year interval with 8.9% (95% CI 2.6–26.8%). Finally, based on country distribution, Tunisia registered the highest PPE at 37.3% while the lowest was observed in Nigeria at 15.4% (95% CI 6.2–33.5%) (Table 4).
Table 4. Subgroup analysis of Giardia species contamination in waterbodies across Africa

Risk of publication bias of included studies
The funnel plots of the estimates suggested publication bias (Supplementary Figs S1–S8) for both human studies with asymmetric presentations and BMR test values observed with respect to human studies were microscopy method (P = 0.000), males (P = 0.004), females (P = 0.008), 2011–2022 year interval (P = 0.000), HIV+ (P = 0.004), HIV– (P = 0.007), diarrhoeic (P = 0.001), Cameroon (P = 0.014), Ethiopia (P = 0.000), Nigeria (P = 0.000) and Sudan (P = 0.025). Whereas for animals, observed publication bias was on 2001–2010 year interval (P = 0.040), cattle (P = 0.009) and Ethiopia (P = 0.019) (Tables 3 and 4 and Supplementary Figs S9–S11). However, Funnel plots and BMR tests suggested no publication bias for waterbody studies (Fig. 2).
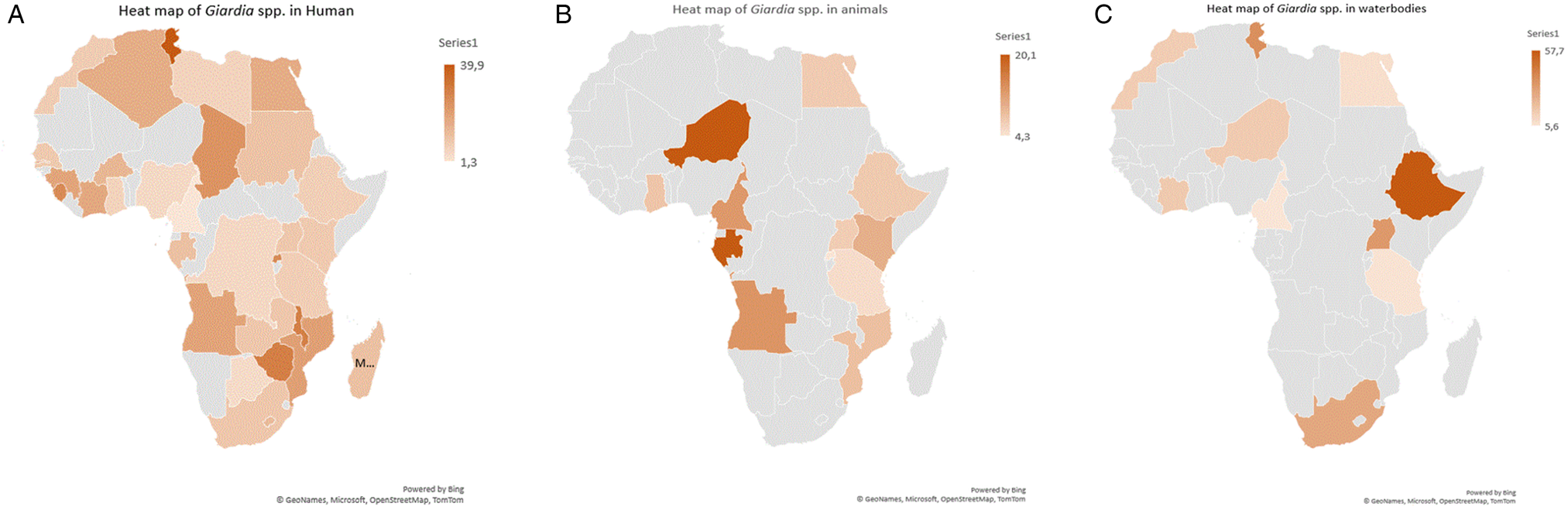
Figure 2. Heat maps showing pooled prevalence estimates of Giardia spp. per country (A) humans, (B) animals and (C) waterbodies.
Discussion
Herein, we carried out a robust study to investigate the epidemiology and occurrence of Giardia species in humans, animals and from waterbodies across Africa using over 500 published articles that employ different diagnostic methods including microscopy, copro-antigen and molecular-based techniques. Arising from differences in the sensitivity and specificity of the diagnostic methods employed in the investigation of the prevalence of giardiasis, we observed varying prevalence rates (Hooshyar et al., Reference Hooshyar, Rostamkhani, Arbabi and Delavari2019). Light microscopy is regarded as the gold standard method recommended for the diagnosis of cystic and/or trophozoite stages of G. duodenalis (Soares and Tasca, Reference Soares and Tasca2016). Several factors including number of fecal samples and operator experience examined may affect the outcome of investigation of giardiasis using this approach as opined by Elmi et al. (Reference Elmi, Gholami, Rahimi-Esboei, Garaili, Najm and Tabatabaie2017) and David and AP (Reference John and Petri2006) leading to lower prevalence of infection or complete failure of detection (Taghipour et al., Reference Taghipour, Sharbatkhori, Tohidi, Ghanbari, Karanis, Olfatifar, Majidiani, Khazaei, Bahadory and Javanmard2022). There is an increased utilization of immunodiagnostic techniques to complement fecal microscopy for giardiasis detection and appears to be reliable for confirmatory diagnosis (Hooshyar et al., Reference Hooshyar, Rostamkhani, Arbabi and Delavari2019). On the other hand, DNA-based molecular methods are also reported to be more reliable with appropriate sensitivity for the identification of Giardia species (Thompson and Monis, Reference Thompson and Monis2004; Gelanew et al., Reference Gelanew, Lalle, Hailu, Pozio and Cacciò2007).
Giardia species infection in humans
The total PPE of Giardia parasite infections in humans was higher using molecular techniques (19.5%) as compared to microscopic (8.8%) and copro-antigen (14.3%), a finding that is similar to that documented in Ghana and Spain, respectively, where molecular tests (6.6 and 37.5%) detected more Giardia spp.-positive infections than microscopy (1.0 and 4.1%) and copro-antigen (5.6 and 4.1%) (Anim-Baidoo et al., Reference Anim-Baidoo, Narh, Oddei, Brown, Enweronu-Laryea, Bandoh, Sampane-Donkor, Armah, Adjei, Adjei, Ayeh-Kumi and Gyan2016; Alharbi et al., Reference Alharbi, Toulah, Wakid, Azhar, Farraj and Mirza2020). However, Al-Shehri et al. (Reference Al-Shehri, Stanton, LaCourse, Atuhaire, Arinaitwe, Wamboko, Adriko, Kabatereine and Stothard2016) in Uganda (41.6; 19.3; 0%), Gasparinho et al. (Reference Gasparinho, Ferreira, Mayer, Mirante, Vaz Nery, Santos-Reis, Portugal-Calisto and Brito2017) in Angola (18.8; 4.1; 0.5%) and Jelinek and Neifer (Reference Jelinek and Neifer2013) in Germany (51.1; 50.0; 46.6%) reported that serological tests detected more positives as compared to microscopic and molecular techniques in that order. Whereas in Gambia, Goudal et al. (Reference Goudal, Laude, Valot, Desoubeaux, Argy, Nourrisson, Pomares, Machouart, Le Govic, Dalle and Botterel2019) and Sullivan et al. (Reference Sullivan, Neale, Cevallos and Farthing1991) reported that microscopy (20.1 and 48.6%) detected more Giardia spp.-positive infections as compared to serological tests (18.5 and 30.6%) in that order respectively. Furthermore, Emisiko et al. (Reference Emisiko, Shaviya, Shiluli, Wamalwa, Jumba, Zablon, Mambo and Barasa2020) in Kenya (46.5; 13.0%), Geus et al. (Reference Geus, Sifft, Habarugira, Mugisha, Mukampunga, Ndoli, Bayingana, Sendegeya, Martus, Fraundorfer and von Samson-Himmelstjerna2019) in Rwanda (36.0; 7.1%) and El Fatni et al. (Reference El Fatni, Olmo, El Fatni, Romero and Rosales2014) in Morocco (12.5; 3.0%) reported that microscopy detected more Giardia spp.-positive infections as compared to molecular methods. Remarkably, Becker et al. (Reference Becker, Chatigre, Gohou, Coulibaly, Leuppi, Polman, Chappuis, Mertens, Herrmann, N'goran and Utzinger2015) reported similar detection prevalence (28.7%) by microscopy, copro-antigen and molecular methods in Côte d'Ivoire. Van den Bossche et al. (Reference Van den Bossche, Cnops, Verschueren and Van Esbroeck2015) (4.1; 4.1%) in Belgium and Doni et al. (Reference Doni, Zeyrek, Gürses and Tümer2013) (19.6; 19.6%) in Turkey reported equal prevalence on microscopic and copro-antigen methods and Irisarri-Gutiérrez et al. (Reference Irisarri-Gutiérrez, Hernández-de Mingo, de Lucio, Gil, Morales, Seguí, Nacarapa, Muñoz-Antolí, Bornay-Llinares, Esteban and Carmena2017) in Mozambique (6.1; 7.1%) and El-Badry et al. (Reference El-Badry, Ghieth, Ahmed and Ismail2017) in Egypt (11.0; 11.9%) reported microscopic and copro-antigen methods to have similarly equal results towards molecular methods, respectively. This difference can be accounted by differences in specificity and sensitivity of detection techniques used and our review showed that prevalences generally are higher when molecular detection methods are used compared to microscopic or copro-antigen tests, suggesting that molecular tests have a higher sensitivity.
Country-specific findings indicate that Tunisia had the highest PPE of 39.9% for Giardia spp. infections, with the rural populations (17.9%) being more susceptible as compared to the population in urban regions (9.2%). This observation is similar to that reported by Díaz et al. (Reference Díaz, Salazar and Valle2015) in Peru (53.1% rural; 40.6% urban), El Fatni et al. (Reference El Fatni, Olmo, El Fatni, Romero and Rosales2014) in Morocco (19.6% rural; 7.6% urban), Cervantes Gracia et al. (Reference Cervantes Gracia, Llanas-Cornejo and Husi2017) in Mexico (22.3% rural; 17.9% urban), Heimer et al. (Reference Heimer, Staudacher, Steiner, Kayonga, Havugimana, Musemakweri, Harms, Gahutu and Mockenhaupt2015) in Rwanda (43.9% rural; 8.7% urban) and Lobo et al. (Reference Lobo, Augusto, Antunes, Ceita, Xiao, Codices and Matos2014) in DR Congo (18.7% rural; 1.9% urban). In contrast, Huot et al. (Reference Huot, Mom, Sean, Supaprom, Rachmat, Luy, Bunnan, Sopheab and Dejli2016) and Loewenson et al. (Reference Loewenson, Mason and Patterson1986) reported higher prevalence of Giardia parasite infections in peri-urban regions (12.3; 22.3%) as compared to rural regions (7.2; 15.6%) in Cambodia and Zimbabwe in that order respectively. However, Ahmad et al. (Reference Ahmad, El-Kady and Hassan2020), Berrilli et al. (Reference Berrilli, Di Cave, D'orazi, Orecchia, Xhelilaj, Bejko, Caca, Bebeci, Cenko, Donia and Divizia2006), Ngonjo et al. (Reference Ngonjo, Kihara, Njoka, Gicheru, Wanzala and Mwandawiro2015) and Roche and Benito (Reference Roche and Benito1999) reported similar Giardia spp. prevalence between urban (20.8; 44; 6.9; 7.2%) and rural (21.6; 44; 7.4; 8.6%) area populations in Egypt, Albania, Kenya and Guinea-Bissau, respectively. About 40.0% of the population in Africa resides in urban areas and thus, anthropogenic activities might be responsible for this observation. Furthermore, the wide variety of socio-economical, climate and geographical characteristics might have influenced this geographical difference in Giardia species prevalence (Ahmed et al., Reference Ahmed, Guerrero Flórez and Karanis2018). The infection was higher in male (13.3%) subjects compared to females (11.9%). Similarly, Abdel-Aziz et al. (Reference Abdel-Aziz, Afifi, Malik and Adam2010), Anim-Baidoo et al. (Reference Anim-Baidoo, Narh, Oddei, Brown, Enweronu-Laryea, Bandoh, Sampane-Donkor, Armah, Adjei, Adjei, Ayeh-Kumi and Gyan2016), Bauhofer et al. (Reference Bauhofer, Cossa-Moiane, Marques, Guimaraes, Munlela, Anapakala, Chilaule, Cassocera, Langa, Chissaque and Sambo2020), Dacal et al. (Reference Dacal, Saugar, de Lucio, Hernández-de-Mingo, Robinson, Köster, Aznar-Ruiz-de-Alegría, Espasa, Ninda, Gandasegui and Sulleiro2018) and Díaz et al. (Reference Díaz, Salazar and Valle2015) indicated that Giardia spp. infections were more common in males (37.8; 7.1; 10.5; 43.0; 51.5%) as compared to females (28.0; 3.9; 8.5; 34.2; 35.8%) in Sudan, Ghana, Mozambique, Angola and Peru, respectively. Akinbo et al. (Reference Akinbo, Okaka and Omoregie2010), Bayoumy et al. (Reference Bayoumy, Ibrahim, Abou El Nour and Said2016), Júlio et al. (Reference Júlio, Vilares, Oleastro, Ferreira, Gomes, Monteiro, Nunes, Tenreiro and Ângelo2012) and Kasaei et al. (Reference Kasaei, Carmena, Jelowdar and Beiromvand2018) reported similar prevalence of Giardia spp. among males (0.0; 3.9; 6.9; 50%) and females (0.1; 3.8; 6.5; 50%) in Portugal and Iran, respectively. However, in Uganda, South Africa, Ethiopia and Turkey, the prevalence of Giardia spp. was lower (15.9; 32.5; 13.7;18.2%) for males and (22.7; 67.5; 19.8; 20.8%) for females (Ali et al., Reference Ali, Mekete and Wodajo1999; Jarmey-Swan et al., Reference Jarmey-Swan, Bailey and Howgrave-Graham2001; Doni et al., Reference Doni, Zeyrek, Gürses and Tümer2013; Al-Shehri et al., Reference Al-Shehri, Stanton, LaCourse, Atuhaire, Arinaitwe, Wamboko, Adriko, Kabatereine and Stothard2016). Environmental risk factors such as work and sports activities may potentially be contributing on the higher male prevalence of Giardia spp. in the African continent.
Our results also revealed that the Giardia spp. infection was predominantly among children <6 months to 18 years with PPE of 13.4%, compared to adults >36 years (PPE 9.4%). Our findings corroborate observations from different researchers from different countries as documented by Belkessa et al. (Reference Belkessa, Ait-Salem, Laatamna, Houali, Sönksen, Hakem, Bouchene, Ghalmi and Stensvold2021) in Algeria (81.8%), Casalino et al. (Reference Casalino, Yusuf, Nicoletti, Bazzicalupo, Coppo, Colonna, Cappelli, Bianchini, Falbo, Ahmed and Omar1988) in Somalia (4.1%), El-Mohammady et al. (Reference El-Mohammady, Mansour, Shaheen, Henien, Motawea, Raafat, Moustafa, Adib-Messih, Sebeny, Young and Klena2012) in Egypt (19.1%) and Rafiei et al. (Reference Rafiei, Baghlaninezhad, Köster, Bailo, Hernández de Mingo, Carmena, Panabad and Beiromvand2020) in Iran (12.7%) where they reported that children <10 to 20 years were more susceptible than other age groups. On the contrary, Berhe et al. (Reference Berhe, Bugssa, Bayisa and Alemu2018) and Esrey et al. (Reference Esrey, Collett, Miliotis, Koornhof and Makhale1989) in Ethiopia and Lesotho, respectively, registered higher Giardia spp. prevalence in adults aged 19–35 years compared to other age groups. The high prevalence in children can be attributed to unhygienic practices, which include not washing their hands, biting their nails and walking barefoot (Molina et al., Reference Molina, Pezzani, Ciarmela, Orden, Rosa, Apezteguía, Basualdo and Minvielle2011). We observed a 3.5% decline of Giardia spp. infections in humans between 1980–1990 and 1991–2000 which was followed by an increase of 1.7% during 1991–2000 and 2001–2010. These periodic fluctuations across the continent suggest possible inconsistencies in the personal hygiene, exposure to infected animals, consumption of contaminated food and water, as well as inadequate surveillance of Giardia parasite infections.
Furthermore, this study found that HIV+ individuals (5.0%) were more infected with Giardia spp. as compared to HIV– subjects (4.1%). These findings concur with the reports by Babatunde et al. (Reference Babatunde, Salami, Fabiyi, Agbede and Desalu2010), Bailey et al. (Reference Bailey, Thomas, Green, Bailey and Beeching2006), Feitosa et al. (Reference Feitosa, Bandeira, Sampaio, Badaró and Brites2001), Liu et al. (Reference Liu, Xu, Shen, Hu and Cao2019) and Oguntibeju (Reference Oguntibeju2006) where they recorded higher prevalence of Giardia parasite infections in HIV+ (17.7, 5.3; 4.9; 2.8; 16.7%) as compared to HIV– (5.6; 0.8; 2.4; 0; 10.0%) individuals in Nigeria, South Africa, Brazil, Guangxi and Lesotho, respectively. In contrast, in Guinea-Bissau, Honduras and Senegal, the Giardia parasite infection prevalence was slightly higher in HIV– (20.0; 12.5; 2.9%) as compared to HIV+ (8.6; 1.9; 1.7%) patients (Lindo et al., Reference Lindo, Dubon, Ager, de Gourville, Solo-Gabriele, Klaskala, Baum and Palmer1998; Gassama et al., Reference Gassama, Thiaw, Dia, Fall, Camara, Hovette, Perret, Gueye-Ndiaye, Mboup, Sow and Aidara-Kane2001; Roka et al., Reference Roka, Goñi, Rubio and Clavel2012). It is well known that HIV-infected individuals have immuno-compromised system due to loss of CD4 T cells, making them more vulnerable to a variety of illnesses (Faria et al., Reference Faria, Zanini, Dias and do Céu Sousa2017) including opportunistic Giardia parasite.
The current study revealed that diarrhoeic individuals (12.3%) had higher Giardia spp. infection prevalence than non-diarrhoeal individuals (9.7%). Our findings agree with the report from Algeria (14.6 and 0.3%), India (16.0 and 8.0%), Libya (26.3 and 0.0%) and Madagascar (12.6 and 7.7%) where diarrhoeic individuals had higher Giardia parasite infection prevalence as compared to non-diarrhoeal individuals (Dwivedi et al., Reference Dwivedi, Prasad, Saini, Mahajan, Lal and Baveja2007). In contrast, Bodhidatta et al. (Reference Bodhidatta, McDaniel, Sornsakrin, Srijan, Serichantalergs and Mason2010), Haque et al. (Reference Haque, Roy, Kabir, Stroup, Mondal and Houpt2005), Messa et al. (Reference Messa, Köster, Garrine, Gilchrist, Bartelt, Nhampossa, Massora, Kotloff, Levine, Alonso and Carmena2021) and Tellevik et al. (Reference Tellevik, Moyo, Blomberg, Hjøllo, Maselle, Langeland and Hanevik2015) found that Giardia spp. prevalence was higher in non-diarrhoeal (10.0; 18.0; 32.0; 6.1%) as compared to diarrhoeal (6.0; 7.7; 20.0; 3.4%) individuals in Thailand, Bangladesh, Mozambique and Tanzania, respectively. However, some studies reported similarly equal prevalence on diarrhoea and non-diarrhoea in DRC (2.3 and 1.7%) and Nigeria (0.5 and 0.0%), respectively (Ogunsanya et al., Reference Ogunsanya, Rotimi and Adenuga1994; Wumba et al., Reference Wumba, Longo-Mbenza, Mandina, Wobin, Biligui, Sala, Breton and Thellier2010). These differences can be attributed to variable immune responses of individuals.
Giardia spp. infection in animals
This study has recorded PPE of 17.7% for detection of Giardia infections in animals using serological technique which is higher than microscopy and molecular methods. Fayer et al. (Reference Fayer, Santin and Macarisin2012) reported a higher prevalence of 51.1% using molecular technique compared to microscopic methods (19.2%) in the USA. Furthermore, the study by Wang et al. (Reference Wang, Wang, Li, Zhang, Karanis, Jian, Ma and Karanis2018) found that both serology (5.8%) and molecular (5.2%) methods had similarly higher detection performance as compared to microscopy (3.7%) for detection of Giardia spp. infections in China. On the contrary, Bouzid et al. (Reference Bouzid, Halai, Jeffreys and Hunter2015) reported higher Giardia spp. infections by microscopy (53.3%) in comparison to molecular (9.2%) and serological methods (26.6%) in Norwich and UK. This difference in diagnostic methods might be due to individual assay sensitivity and less expertise in microscopy.
The subgroup analysis at the country level showed that the PPE of Giardia spp. in different African countries ranged from 4.3 to 20.1% with the highest from Nigeria and lowest in Rwanda. These differences might be due to sample size, methodology used to detect and number of studies included in the current study. Animal species were grouped into domestic (cats, cattle, dogs, goats, pigs and sheep) and wildlife (baboons, bonobos, buffaloes, bushbuck, chimpanzee, grasscutter, gorillas, guenons, lions, Maxwell's duiker, monkeys, rabbit, rat, royal antelopes and wild dogs) with each group prevalence pooled together. Our subgroup analysis also revealed that wildlife had lower PPE as compared to domestic animals. However, Castro-Hermida et al. (Reference Castro-Hermida, García-Presedo, González-Warleta and Mezo2011) reported wildlife as the main source of exposure for the transmission of Giardia parasite to domestic animals, humans, as well as contamination of waterbodies. Our study witnessed a 13.6% increase in the continent PPE of Giardia pathogen in animals during the period of 1990–2022. However, periodic analysis revealed a 6.3% initial increase between 1990–2000 and 2001–2010 intervals which was followed by a continuous increase of 7.3% during the 2001–2010 and 2011–2022 intervals. This continuous increase of Giardia pathogens might be attributed to failure of animal disease control programmes across the African continent and the use of more advanced diagnostic techniques as years go by.
Giardia spp. infection in waterbodies
Before the year 2000, little interest was shown from researchers within the continent to investigate the prevalence and distribution of Giardia spp. in Africa. Interest began to build in the last 2 decades and may be connected to increased resistance of the parasite to chemicals used for water treatment (Jarroll et al., Reference Jarroll, Muller, Meyer and Morse1981; Rice et al., Reference Rice, Hoff and Schaefer1982; Gerba et al., Reference Gerba, Abbaszadgan, Hasan and Phillips1993). The prevalence of Giardia pathogens measured by molecular methods was higher than microscopic-based methods for most of the environmental waterbodies and varied across countries, with the highest in Tunisia 37.27% and the lowest in Nigeria 15.4%. A study in India has documented equivalent prevalence using molecular (32.0%) and microscopic methods (31.3%) in rivers (Roy et al., Reference Roy, Singha, Dhar and Roychoudhury2019). However, high prevalence of Giardia infection using microscopic method (100.0%) compared to molecular methods (96.2%) in municipal and domestic wastewater in Iran has also been reported (Hatam-Nahavandi et al., Reference Hatam-Nahavandi, Mohebali, Mahvi, Keshavarz, Mirjalali, Rezaei, Meamar and Rezaeian2017). This was attributed to the inability of molecular methods to differentiate between viable and non-viable DNA of Giardia parasites and lack of expertise in the use of microscope to identify the parasite.
‘One Health’ perspective
The ‘One Health’ approach seeks to develop cross-disciplinary relationships to provide more comprehensive responses to diseases that affect multiple species (Lerner and Berg, Reference Lerner and Berg2015). A ‘One Health’ concept is becoming more and more necessary, especially in Africa where there is a challenge of access to clean water, and humans living in close contact with animals in rural settlements (Collignon and McEwen, Reference Collignon and McEwen2019).
This study has reported a consolidated Giardia spp. infection prevalence in non-human primates (NHPs), domestic animals, wildlife and waterbodies of the African continent. Furthermore, this study has shown that there is high prevalence of Giardia spp. in rural settlements. It is well known that majority of African rural communities practice extensive communal farming which is mainly at livestock–wildlife interface where cattle, goats, pigs and sheep often share pastures and waterbodies with wildlife. In some cases, humans also share the same water sources with animals, hence, the ‘One Health’ concern. Our results highlight the significance of ‘One Health’ concept to comprehend the epidemiology of giardiasis as our study has highlighted the intricate link of the pathogen with human health, the health of both domestic and wild animals, as well as the integrity status of waterbodies. For instance, in China, G. duodenalis has been found in large numbers in humans, NHPs, domestic animals, pet animals, wildlife, as well as the environment (Wang et al., 2017; Li et al., Reference Li, Qin, Li and Zhang2023). Only investigations using One Health strategies, which simultaneously consider humans, domestic animals, wildlife and waterbodies, will provide a clear understanding of the key routes of transmission for Giardia spp. in Africa. This qualifies giardiasis as one of the diseases in Africa that requires ‘One Health’ approach.
Conclusion and limitations
Findings of this study suggest that rural population, males, children up to 18 years age, diarrhoeal and HIV+ individuals were subgroups at high risk of getting infected by Giardia spp. Whereas for animal demographics, domestic animals were subgroups at high risk of getting infected with Giardia spp. We further observed that Giardia spp. were also prevalent in NHPs such as chimpanzees, gorillas and monkeys. The robust data presented in this study can be helpful to doctors, veterinarians and environmental scientists by informing them about the epidemiological status of Giardia pathogens in humans, animals and waterbodies in Africa.
Our study did not examine the assemblage's diversity of Giardia pathogens in humans, animals and waterbodies. Moreover, we only included studies that were published in English language and this language bias has possibly resulted in omission of some relevant studies published in other languages. There were no studies available for Giardia parasite infections in Burundi, Cabo Verde, Central African Republic, Congo, Djibouti, Equatorial Guinea, Eswatini, Guinea, Liberia, Mauritania, Mauritius, Niger, Seychelles and South Sudan for humans. In animal studies, some important variables such as sex, stool consistency and age of animals were lacking in included studies. Only 1 study was included in 1980–1990 study interval and there were no studies available in countries such as Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Chad, Comoros, Congo, Djibouti, Equatorial Guinea, Eritrea, Eswatini, Gambia, Guinea, Lesotho Liberia, Libya, Madagascar, Malawi, Mali, Mauritania, Mauritius, Morocco, Niger, Sao Tome and Principe, Seychelles, Sierra Leone, Somalia, South Sudan, Sudan, Togo, Tunisia and Zimbabwe.
In studies involving waterbodies, only 1 study was included in the year 1980 until 2000 and no data were available in countries such as Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cabo Verde, Central African Republic, Chad, Comoros, Congo, Djibouti, DR Congo, Equatorial Guinea, Eritrea, Eswatini, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho Liberia, Libya, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, Somalia, South Sudan, Sudan, Togo and Zambia. Finally, we recommend that future studies should be directed to investigation of the epidemiology of this protozoan parasite in countries where surveillance is low as this parasite may pose danger to animals and citizens of those countries.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182023000513.
Author contributions
Conceptualization, M. T., T. O. and O. T.; methodology, T. R. and M. T.; validation, M. T., T. R. and O. T.; formal analysis, M. T., T. O., T. R. and O. T.; writing original draft preparation, M. T., T. O. and O. T.; writing review and editing, M. T., T. R., T. O. and O. T.; supervision, T. O. and O. T. All authors have read and agreed to the published version of the manuscript.
Financial support
This research work did not receive any specific grant from funding agencies.
Competing interest
None.
Ethical standards
Not applicable.