Article contents
The Hydrolysis of Ammonium Ions in Sea Water-a Theoretical Study
Published online by Cambridge University Press: 11 May 2009
Extract
A number of procedures are now available for calculating the effects of ionic inter-actions on the behaviour of solutions as complex as sea water (Whitfield, 1973 a). These procedures are able to give a good account of the properties of the major electrolyte components (Leyendekkers, 1973 a; Robinson & Wood, 1972; Whitfield, 19736) and of the colligative properties of sea water (Robinson & Wood, 1972; Whitfield, 1973 c, d). However, greatest interest centres around the possibility of predicting the effects of these major components on the multitude of ionic equilibria that influence the properties of the less abundant constituents that are of greater biological and geological importance. It is here that the newer approaches to marine chemistry are weakest because suitable thermodynamic data are lacking. One system of practical importance that can be studied using a variety of theoretical approaches is the acid ionization of ammonium ions represented by the equation 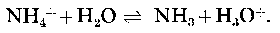 The toxicity of ammonium salts to freshwater life has been shown to be strongly dependent on the pH in a manner that is consistent with un-ionized ammonia (NH3) being the most lethal fraction (see, for example, Wuhrmann & Woker, 1948; Downing & Merkens, 1955; Lloyd & Herbert, i960; Hemens, 1966; and Brown, 1968). These papers and many others have been thoroughly reviewed (EIFAC Technical Paper no. 11, 1970, Kemp, Abrams & Overbeck, 1971). The free base (NH3) has a relatively high lipid solubility because it carries no charge and is therefore able to diffuse quite readily across cell membranes (Fromm & Gillette, 1968).
The toxicity of ammonium salts to freshwater life has been shown to be strongly dependent on the pH in a manner that is consistent with un-ionized ammonia (NH3) being the most lethal fraction (see, for example, Wuhrmann & Woker, 1948; Downing & Merkens, 1955; Lloyd & Herbert, i960; Hemens, 1966; and Brown, 1968). These papers and many others have been thoroughly reviewed (EIFAC Technical Paper no. 11, 1970, Kemp, Abrams & Overbeck, 1971). The free base (NH3) has a relatively high lipid solubility because it carries no charge and is therefore able to diffuse quite readily across cell membranes (Fromm & Gillette, 1968).
- Type
- Research Article
- Information
- Journal of the Marine Biological Association of the United Kingdom , Volume 54 , Issue 3 , August 1974 , pp. 565 - 580
- Copyright
- Copyright © Marine Biological Association of the United Kingdom 1974
References
REFERENCES
- 132
- Cited by




