Inflammation is a complex immune response to pathogenic agents(Reference Actor and Smith1). Indeed, both cell-mediated and humoral responses are involved in inflammation(Reference Brenner, Scherer and Muir2), whilst reactive oxygen species are key molecules that play a major role in the initiation and progression of the inflammatory response(Reference Chelombitko3).
Inflammation may be classified into two types: acute and chronic(Reference Pahwa and Jialal4). Acute inflammation is a short-term immune response to detrimental conditions, such as tissue injury, and can facilitate repair, turnover and adaptation of tissues(Reference Howcroft, Campisi and Louis5). Although chronic inflammation has many characteristics of acute inflammation, it is usually mild and permanent(Reference Franceschi and Campisi6). Although chronic inflammation is not considered as a separate disease, several chronic diseases have an inflammation-based pathogenesis pain. Accumulating evidence suggests that diabetes(Reference Sena, Carrilho and Seiça7), CVD(Reference Maffia and Cirino8), cancer(Reference Munn9), obesity(Reference Ellulu, Patimah and Khaza’ai10), rheumatoid arthritis(Reference Gravallese11) and chronic respiratory diseases(Reference Di Gioia, Sardo and Castellani12) are all associated with inflammation.
Lifestyle modification, including adopting a healthy diet(Reference Akbaraly, Shipley and Ferrie13), regular exercise(Reference Gómez-Rubio and Trapero14), adequate sleep(Reference Kinnucan, Rubin and Ali15), avoiding smoking(Reference Rom, Avezov and Aizenbud16) and stress management(Reference Parker, Smarr and Buckelew17), can reduce chronic inflammation. Adequate intake of vegetables and legumes is regarded as an important part of a healthy diet(Reference Gilham, Hall and Woods18). Soya beans are legumes, rich in health-promoting components such as vitamin E, vitamin C, folates, thiamin, riboflavin, amino acids and bioactive compounds(Reference Martino, Cardoso and Ribeiro19), whilst, to our knowledge, soyabean protein possesses antioxidant, anti-inflammatory and anticancer properties(Reference Gao, Wang and Yuan20). In addition to minerals, vitamins, fibre and n-3 fatty acids, soya beans are considered as a major source of phyto-oestrogens, particularly isoflavones(Reference Jooyandeh21,Reference Greaves, Wilson and Rudel22) . Genistein, daidzein and glycitein are the major isoflavones found in soya beans(Reference Greaves, Wilson and Rudel22). Genistein has anti-inflammatory properties and is a strong inhibitor of tyrosine kinase enzyme(Reference Verdrengh, Jonsson and Holmdahl23), leading to, in part, the suggestion that soyabean intake may be efficacious in the prevention and treatment of inflammation-based chronic diseases(Reference Jooyandeh21).
Some studies have reported that soya consumption reduced some inflammatory biomarkers(Reference Amanat, Eftekhari and Fararouei24,Reference Zhang, Wang and Ma25) ; however, equivocally, soya intake had null(Reference Napora, Short and Muller26,Reference Rebholz, Reynolds and Wofford27) or unfavourable(Reference Lebon, Riesco and Tessier28) effects on inflammation in other studies. Therefore, a systematic review and meta-analysis is needed to determine the overall effect of soya consumption on inflammatory biomarkers. Although a previous meta-analysis reported that soya consumption had no significant effect on C-reactive protein (CRP)(Reference Khodarahmi, Jafarabadi and Moludi29), there is no comprehensive systematic review and meta-analysis of clinical trials that has evaluated the impact of soya intake on other inflammatory markers. Therefore, the purpose of this systematic review and meta-analysis was to determine the effects of soya and soya products on inflammatory biomarkers.
Methods
The present study was conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis(Reference Moher, Liberati and Tetzlaff30). The study protocol was registered on an international prospective register of systematic reviews (PROSPERO) (registration number: CRD42020164481).
Search strategy
Electronic databases, including Medline, Scopus, ISI Web of Science and Google Scholar, were searched up to and including May 2020. Title, abstract and keywords of articles were searched using the following keywords: (‘soya’ or ‘soy foods’ or ‘soy milk’ or ‘soybeans’ or ‘soybean protein’ or ‘soy’ or ‘isoflavones’ or ‘phytoestrogens’ or ‘genistein’ or ‘genestein’ or ‘glycitein’ or ‘daidzein’ or ‘isolated soy protein’ or ‘textured soy protein’) AND (‘interleukin-6’ or ‘IL-6’ or ‘tumor necrosis factor-α’ or ‘TNF-α’ or ‘interleukin’ or ‘interleukin-8’ or ‘inflammation’ or ‘cytokine’ or ‘IL-1β’ or ‘IL-2’ or ‘IL-4’ or ‘IL-8’ or ‘IL-10’ or ‘IFN-γ’ or inflammatory’). The references of the retrieved articles were also searched manually. The search strategy was conducted without any restrictions.
Eligibility criteria
Two independent investigators (M. R. and F. M.) screened title, abstract and full texts of included articles. All interventions that investigated the effects of soya and soya products on inflammatory biomarkers including TNF-α, IL-6, IL-2, IL-1β or interferon γ (IFN-γ), in healthy and unhealthy adults, were included. Articles were excluded if they: (1) were in vitro or animal-based studies; (2) were editorials, letters, review articles or meeting abstracts; (3) were short-term (<1 week); (4) used soya in combination with other foods or adjunct interventions; (5) had no control group; (6) did not report dose of soya or isoflavone in intervention group; (7) reported post-exercise inflammation; (8) included pregnant women or children and (9) had insufficient reported data.
Data extraction
The following information was extracted from each eligible article: the first author’s name and year of publication; sample size; age of participants; design of clinical trial and duration; dosage and type of soya or soya product used in the intervention group; details regarding intervention in the control group and characteristics of subjects. IL-6, TNF-α, IL-2, IL-10, IL-1β and IFN-γ were considered as main outcomes. Mean and standard deviation or standard error for outcomes were extracted. CRP was not entered in the present study because a previous meta-analysis reported the effect of soya consumption on CRP(Reference Khodarahmi, Jafarabadi and Moludi29).
Assessment of quality
The quality of studies was assessed according to the Cochrane Risk of Bias Tool(Reference Higgins, Altman and Gøtzsche31). Two authors (M. R. and F. M.) independently evaluated the quality of eligible studies through Cochrane Risk of Bias tool including seven domains: (1) random sequence generation (selection bias), (2) allocation concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective reporting (reporting bias) and (7) other sources of bias. Each domain was classified into three classes: low risk (one plus (+) sign), high risk (one negative (–) sign) and unclear risk of bias (question mark (?)). Therefore, the overall quality of each study was considered as good (low risk for more than two domains), fair (low risk for two domains) or weak (low risk for less than two domains).
Statistical analysis
This meta-analysis was conducted using STATA software (version 11, Stata Corporation). A limited number of studies reported net change; thus, to calculate effect size in studies that net change was not reported in the soya and control group, we used mean values and standard deviations/standard errors or medians and interquartile ranges(Reference Luo, Wan and Liu32,Reference Wan, Wang and Liu33) . To compute the overall effect, we converted standard errors to standard deviations. TNF-α and IL-6 were reported in different units through the studies; therefore, Hedges’ g was used for these variables. In contrast, mean difference was applied for IL-1β, IL-2 and IFN-γ. A random effects model was conducted to calculate pooled effect size for each main outcome. I squared (I 2) and a fixed effects model were used to evaluate inter-study and between-subgroup heterogeneity, respectively. A pre-planned subgroup analysis based on soya type, soya dosage, duration of intervention, design of the study, sex, age and health status was performed to discern potential sources of inter-study heterogeneity.
To evaluate the possible influence of each study on the pooled effect size, the stability of the results was checked using sensitivity analyses. Egger’s regression asymmetry test and Begg’s rank-correlation method were conducted to assess publication bias. A P value <0·05 was considered to represent statistical significance.
Results
Systematic review
Details regarding study selection process are illustrated in Fig. 1. A total of 15 179 records were identified through database searching. Subsequently, 3913 duplicate records were removed, and 11 266 records were screened. After screening, 11 218 records were excluded, and of the forty-eight articles that remained for full-text assessment, nineteen articles were excluded due to being short-term (<1 week) trials (n 3), using soya in combination with other intervention (n 5), using gene expression and cell cultures (n 2), reporting limited data regarding amount of pure soya or isoflavones (n 1), reporting post-exercise inflammation (n 1), being meeting abstract (n 1) and having no control group (n 6). Finally, twenty-nine articles were included in qualitative synthesis(Reference Amanat, Eftekhari and Fararouei24–Reference Lebon, Riesco and Tessier28,Reference Nadadur, Stanczyk and Tseng34–Reference Berg, Schaffner and Pohlmann57) . Whilst, of twenty-nine publications eligible for systematic review, one study was not included in meta-analysis because it did not report applicable data for quantitative analysis, resulting in twenty-eight articles entered into the meta-analysis(Reference Berg, Schaffner and Pohlmann57).

Fig. 1. Flow diagram of the study selection process.
The result of quality assessment of included articles is shown in Table 1. Of the twenty-eight included studies, twenty-five articles were randomised(Reference Amanat, Eftekhari and Fararouei24–Reference Lebon, Riesco and Tessier28,Reference Nadadur, Stanczyk and Tseng34,Reference Christie, Grant and Darnell36–Reference Weiland, Bub and Barth39,Reference Azadbakht, Kimiagar and Mehrabi42–Reference Jalili, Vahedi and Poustchi56) and only sixteen articles reported randomisation methods(Reference Amanat, Eftekhari and Fararouei24–Reference Lebon, Riesco and Tessier28,Reference Nadadur, Stanczyk and Tseng34,Reference Christie, Grant and Darnell36–Reference Weiland, Bub and Barth39,Reference Charles, Yuskavage and Carlson44–Reference Giolo, Costa and Cunha-Junior46,Reference Llaneza, González and Fernandez-Iñarrea48,Reference Ma, Grann and Li50,Reference Jalili, Vahedi and Poustchi56) . Fifteen studies were double-blinded(Reference Amanat, Eftekhari and Fararouei24–Reference Lebon, Riesco and Tessier28,Reference Christie, Grant and Darnell36–Reference Ho, Liu and Loke40,Reference Charles, Yuskavage and Carlson44–Reference Kwak, Ahn and Park47,Reference Jalili, Vahedi and Poustchi56) , and thirteen trials had no report regarding blinding procedure(Reference Nadadur, Stanczyk and Tseng34,Reference Nasca, Zhou and Welty35,Reference Jenkins, Kendall and Connelly41–Reference Beavers, Serra and Beavers43,Reference Llaneza, González and Fernandez-Iñarrea48–Reference Simão, Lozovoy and Bahls55) . Only five articles reported reasons for participant withdrawal(Reference Nasca, Zhou and Welty35,Reference Ho, Liu and Loke40,Reference Jenkins, Kendall and Connelly41,Reference Kwak, Ahn and Park47,Reference Llaneza, Gonzalez and Fernández-Iñarrea49) . Of the twenty-eight included studies in the meta-analysis, the quality of all articles was high, except for two studies which were ranked as low(Reference Nasca, Zhou and Welty35,Reference Jenkins, Kendall and Connelly41) .
Table 1. Cochrane risk of bias assessment
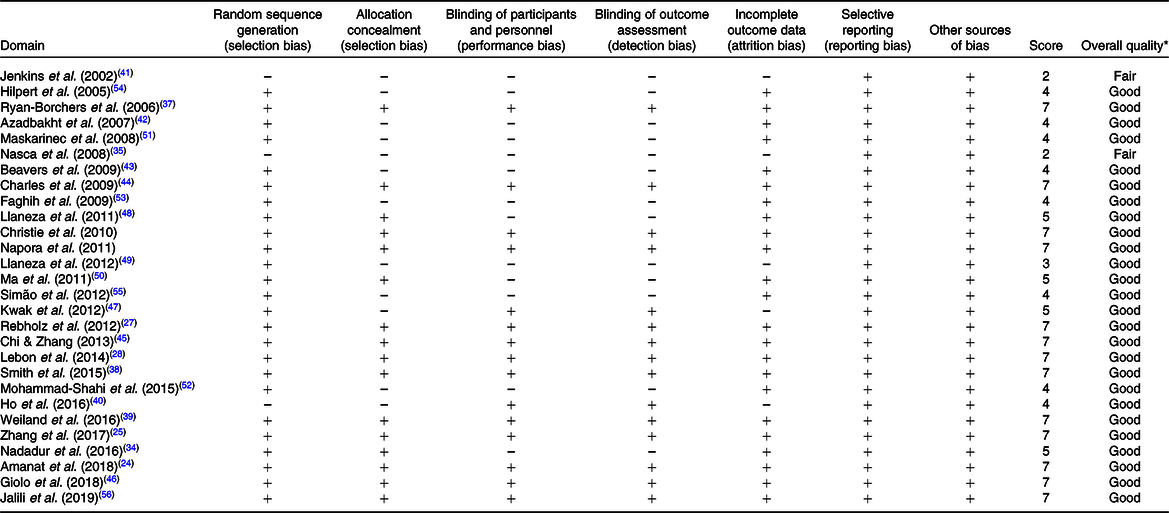
* The overall quality of each study was considered as good (>2 ‘+’ signs), fair (2 ‘+’ signs) or weak (<2 ‘+’ signs).
Details of all twenty-eight articles are presented in Table 2. Twenty-eight clinical trials that enrolled a total of 1816 participants (mean age = 51·4 years) were included in this meta-analysis(Reference Amanat, Eftekhari and Fararouei24–Reference Lebon, Riesco and Tessier28,Reference Nadadur, Stanczyk and Tseng34–Reference Jalili, Vahedi and Poustchi56) . Unhealthy participants had prostate cancer, the metabolic syndrome, irritable bowel syndrome, hypercholesterolaemia, rheumatoid arthritis, climacteric syndrome, Hashimoto’s thyroiditis, poorly controlled asthma, non-alcoholic fatty liver disease and hypertension. Soya was used in different forms through the studies, including soya milk, soya protein, soya nuts and isoflavones. The range of dosage of isoflavones was 40–600 mg, whilst the duration of study varied from 4 to 96 weeks. Twenty studies used a parallel design and eight studies used a crossover design. The most reported outcomes were TNF-α (n 22) or IL-6 (n 21), whilst IL-1β and IL-2 were measured in three studies and IFN-γ level was measured in two studies. IL-10 level was only reported in one study, and therefore, it was only reported in the systematic review.
Table 2. Characteristics of included clinical trials in meta-analysis
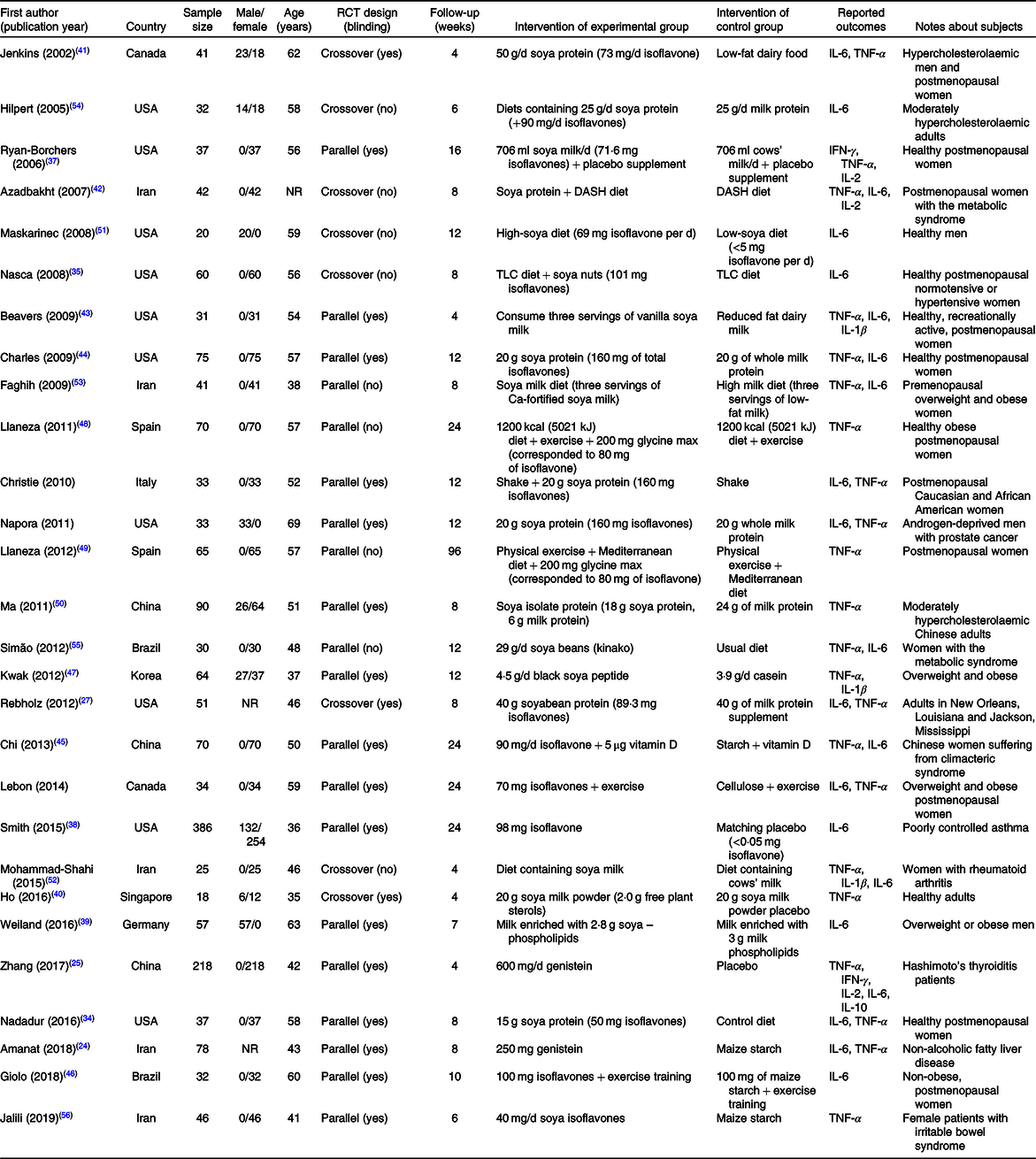
RCT, randomised controlled trial; IFN-γ, interferon γ; NR, not reported; DASH, Dietary Approaches to Stop Hypertension; TLC, therapeutic lifestyle change.
Meta-analysis
The effect of soya and soya products on IL-6
A meta-analysis of twenty-one clinical trials (twenty-three effect sizes) did not yield any significant change in IL-6 level following soya and soya product consumption (Hedges’ g 0·07, 95 % CI –0·14, 0·28) (Fig. 2). There was significant heterogeneity between trials (I 2 = 72 %; P < 0·001); however, we could not discern the sources of heterogeneity by using pre-planned subgroup analysis (Table 3).
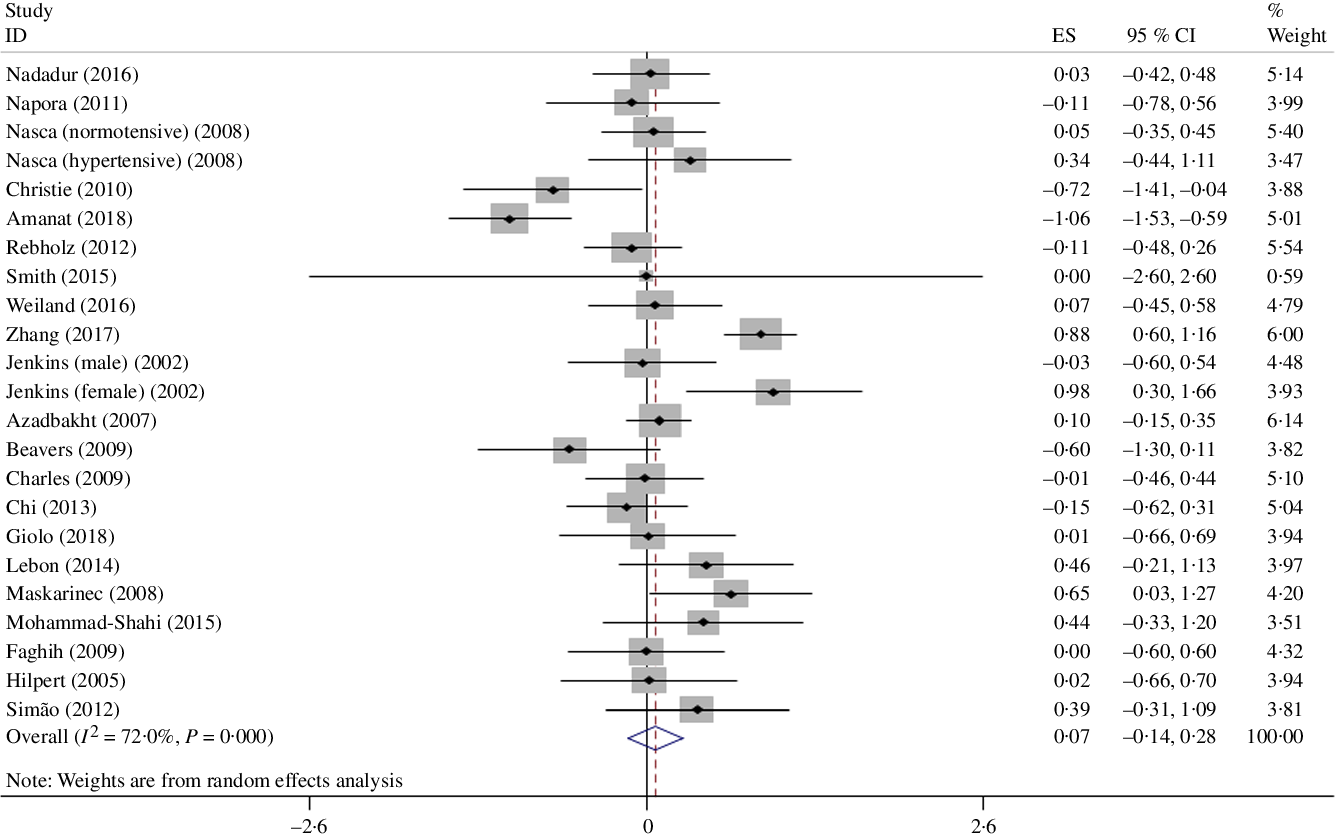
Fig. 2. Forest plot showing the effect of soya consumption on IL-6 level. ES, effect size.
Table 3. Subgroup analysis of included studies in meta-analysis
(Numbers and 95 % confidence intervals)
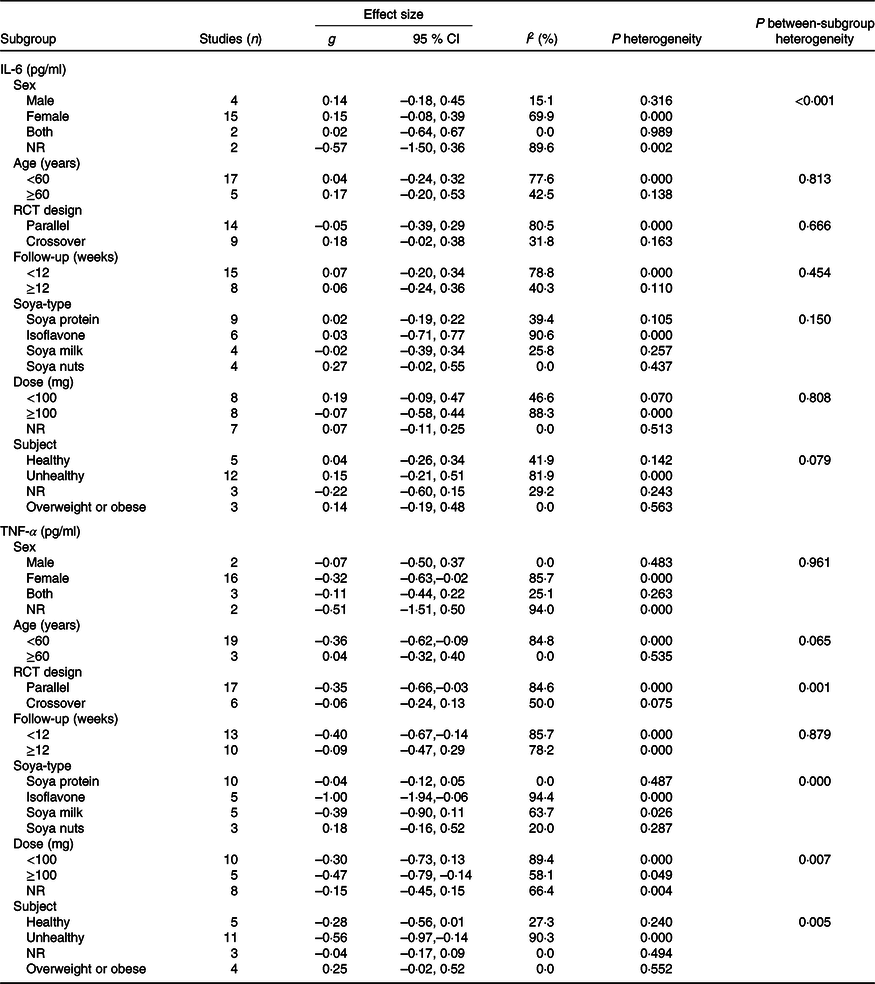
NR, not reported; RCT, randomised controlled trial.
The effect of soya and soya products on TNF-α
The effect of soya intake on TNF-α level was evaluated in twenty-two studies (twenty-three effect sizes). Pooled analysis demonstrated a significant reduction in TNF-α in the soya group compared with controls (Hedges’ g –0·28; 95 % CI –0·49, –0·07). A significant inter-study heterogeneity was identified (I 2 = 82·4 %; P < 0·001), and pre-planned subgroup analysis by soya dosage, design of the study, health status and soya type attenuated the heterogeneity (Fig. 3). As shown in Fig. 3(A), a significant reduction in TNF-α was found in studies that used ≥ 100 mg of isoflavones (Hedges’ g –0·47; 95 % CI –0·79, –0·14; I 2 = 58·1 %). However, we did not observe any significant effect at dosages <100 mg (Hedges’ g –0·30; 95 % CI –0·73, 0·13; I 2 = 89·4 %; P for between-subgroup heterogeneity = 0·007). A significant decrease was shown in parallel designed clinical trials (Hedges’ g –0·35; 95 % CI – 0·66, –0·03; I 2 = 84·6 %); wcontrastingly, crossover studies did not show any significant effect (Hedges’ g –0·06; 95 % CI –0·24, 0·13; I 2 = 50·0 %; P for between-subgroup heterogeneity = 0·001) (Fig. 3(B)). Studies that included unhealthy participants demonstrated a significant reduction in TNF-α (Hedges’ g –0·56; 95 % CI –0·97, – 0·14; I 2 = 90·3 %; P for between-subgroup heterogeneity = 0·005), whilst results in ‘healthy’, ‘overweight or obese’ and ‘not reported’ subgroups were not significant (Fig. 3(C)). Subgroup analysis by soya type further indicated a significant reduction in TNF-α in the studies that used isoflavones supplements (Hedges’ g –1·00; 95 % CI –1·94, –0·06; I 2 = 94·4 %; P for between-subgroup heterogeneity < 0·001), whilst results in ‘soya milk’, ‘soya protein’ and ‘soya nut’ subgroups were not significant (Fig. 3(D)). Further subgroup analyses that could not explain heterogeneity are reported in Table 3.

Fig. 3. Forest plot showing the effect of soya consumption on TNF-α stratified by dosage (A), study design (B), subjects’ health status (C) and soya type (D). ES, effect size.
The effect of soya and soya products on IL-2
Overall effect sizes of three clinical trials (three effect sizes) did not show any significant impact of soya consumption on IL-2 level (mean difference = –1·38 pg/ml; 95 % CI –3·07, 0·31). Although a high inter-study heterogeneity was found (I 2 = 99·6 %; P < 0·001), subgroup analysis was not applicable because of a limited number of studies.
The effect of soya and soya products on IL-1β
Pooled effect sizes of three trials (three effect sizes) did not show any significant effect of soya consumption on IL-1β (mean difference = –0·02 pg/ml; 95 % CI –0·08, 0·03). There was no heterogeneity between studies (I 2 = 0·0 %; P = 0·447).
The effect of soya and soya products on interferon γ
Overall effect sizes of two trials (two effect sizes) did not show a significant effect of soya consumption on IFN-γ level (mean difference = 1685·82 pg/ml; 95 % CI –1604·86, 4976·50). A high inter-study heterogeneity was found (I 2 = 99·8 %; P < 0·001), but subgroup analysis was not carried out because of an insufficient number of studies.
Sensitivity analysis
To evaluate the influence of any individual study on the overall effect size, a sensitivity analysis was performed. For IL-6, TNF-α, IL-2 and IL-1β, excluding any of the studies did not significantly alter the findings. Furthermore, because of the low number of articles, a sensitivity analysis was not carried out for IFN-γ.
Publication bias
Clinical trials did not show publication bias for IL-6 (P = 0·39 for Begg’s test; P = 0·40 for Egger’s test), TNF-α (P = 0·27 for Begg’s test; P = 0·09 for Egger’s test), IL-2 (P = 0·11 for Begg’s test; P = 0·26 for Egger’s test) and IL-1β (P = 0·60 for Begg’s test; P = 0·14 for Egger’s test).
Discussion
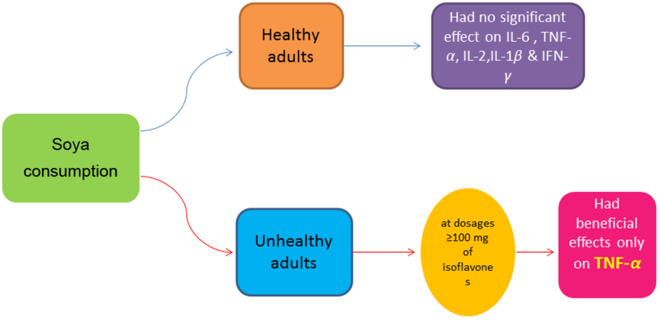
The key findings of this study were that that soya intake had no significant effect on IL-6, IL-1β, IL-2 and IFN-γ, but did yield significant reductions in TNF-α. After conducting subgroup analysis, we found that the beneficial effect of soya intake on TNF-α was only evident in parallel designed studies, at dosages ≥100 mg of isoflavones, and in unhealthy subjects. However, the lack of significant benefit of soya in crossover studies and healthy subjects was likely due to limited power, with only six studies for each subgroup. Indeed, the power of a meta-analysis strongly depends on number of included studies(Reference Turner, Bird and Higgins58). Chronic inflammation, colloquially termed the ‘silent killer’, acts as a strong disease-promoting factor in a variety of disorders, including arteriosclerosis, obesity and cancer(Reference Miyasaka and Takatsu59). Although a review article previously reported the effect of soya and soya product on CRP, there is no systematic review and meta-analysis regarding other inflammatory markers such as TNF-α, IL-6, IL-1β, IL-2 and IFN-γ. A previous meta-analysis reported a non-significant reduction in serum hs-CRP following soya products consumption(Reference Khodarahmi, Jafarabadi and Moludi29), which is comparable with our results regarding IL-6, IL-1β, IL-2 and IFN-γ. In contrast, however, we found that soya intake had a favourable effect on TNF-α. Therefore, it is possible that the effect of soya intake may not be comparable on all inflammatory markers.
In this meta-analysis of clinical trials, we found that soya and soya products consumption had no significant effect on IL-6. However, calculated CI for the effect of soya nut consumption was very close to the significant increase threshold. Indeed, it may be due to the fact that soya nuts are usually consumed in roasted and salted forms. Also, the CI was very close to statistical significance in IL-6 measured in crossover studies. Although crossover studies are more powerful in controlling confounding variables, the number of these studies was low compared with parallel designed studies. Nevertheless, although statistical significance was not formally attained, these results should not be simply overlooked and future studies should further investigate the potential of soya on IL-6.
Our findings showed that, in contrast to ‘healthy’ and ‘overweight or obese’ subgroups, soya intake had a significant effect on reducing TNF-α level in unhealthy subjects. Included studies in the ‘unhealthy’ subgroup enrolled overweight, obese or normal weight participants with prostate cancer, the metabolic syndrome, irritable bowel syndrome, hypercholesterolaemia, rheumatoid arthritis, climacteric syndrome, Hashimoto’s thyroiditis, asthma, non-alcoholic fatty liver disease or hypertension. Studies in the ‘overweight and obese’ subgroup recruited healthy, overweight and obese subjects. A previous study showed that the pattern of inflammation is different between healthy and unhealthy obese subjects(Reference Cătoi, Pârvu and Andreicuţ60), indicating that soya intake may be effective against high levels of TNF-α, as observed in unhealthy, morbidly obese, patients.
We observed that parallel designed studies reported a significant effect of soya intake on TNF-α level. In contrast, however, crossover studies showed no effect. Although crossover studies are more precise in controlling for confounding variables, the number of these studies was low (n 5) compared with parallel designed studies (n 17), highlighting that more crossover studies should be undertaken in this regard.
According to our findings, only soya isoflavones consumption yielded a reduction in TNF-α. Isoflavones are major phyto-oestrogens in soya beans and structurally similar to 17-β-oestradiol(Reference Wang, Ge and Tian61). Genistein, daidzein and glycitein were the types of soya isoflavones used in included studies. The bioavailability of isoflavones is more than other flavonoids(Reference Nielsen and Williamson62); indeed, Ganai et al. reported that genistein reduced nitric oxide and prostaglandin E2 and suppressed production of d-galactosamine-induced proinflammatory cytokines including TNF-α in Wistar rats(Reference Ganai, Khan and Malik63). Moreover, a previous study, in a murine model, showed that daidzein inhibits TNF-α-induced protein poly-adenosine diphosphate-ribosylation(Reference Li, Pan and Ke64). Tanaka et al. illustrated that daidzein suppresses the lipopolysaccharide-induced TNF-α expression(Reference Tanaka, Ohgo and Katayanagi65), whilst genistein can reportedly prevent insulin receptor substrate-1 serine phosphorylation through 5’-adenosine-monophosphate-activated protein kinase(Reference Wang, Gao and Zhao66). Indeed, 5’-adenosine-monophosphate-activated protein kinase activation has a substantial role in anti TNF-α property of genistein(Reference Wang, Gao and Zhao66).
The beneficial effects of soya isoflavones may be related to equol, a specific oestrogenic metabolite of daidzein produced by bacteria in the gut(Reference Jackson, Greiwe and Schwen67). Indeed, some evidence suggests that differences in equol production between humans (between racial/ethnic groups) may explain the reported differences in beneficial effects(Reference Lampe, Karr and Hutchins68). In the present meta-analysis, the conversion of isoflavones to equol was only measured in two studies(Reference Christie, Grant and Darnell36,Reference Ryan-Borchers, Park and Chew37) ; therefore, we were unable to include the conversion of isoflavones to equol in our analysis.
Although soya protein has some beneficial effects on health, high intake or prolonged consumption of soya protein or raw soya beans can be harmful to health. Indeed, soya protein has adverse effects on the endocrine glands, liver and kidney, and elicit carcinogenic effects on the breast, pancreas and thyroid gland(Reference Sukalingam, Ganesan and Das69). Moreover, soya genistein can induce formation of mutagenesis and carcinogenesis, and proliferation of implanted human breast cancer cells(Reference Delclos and Newbold70). Therefore, high consumption of soya and soya products should not be advocated.
The current meta-analysis has some strengths that should considered. The sample size was large because we were able to include twenty-eight articles (1816 participants). Both Egger’s and Begg’s tests indicated no evidence for publication bias. Finally, a comprehensive, pre-defined, subgroup analysis was run. Despite the aforementioned strengths, there are a number of limitations that should be considered: (1) insufficient follow-up duration in some studies and (2) the results of most included studies were not adjusted for confounding factors. However, with regard to these three principle limitations, they were out of the operational control of the study.
Conclusion
In conclusion, the present systematic review and meta-analysis indicated that soya and soya products consumption had no effect on inflammatory biomarkers: IL-6, IL-1β, IL-2 and IFN-γ. A significant reduction was only observed on TNF-α in some specific subgroups. The authors advocate that further, well-controlled, studies should be conducted to clarify the safety and efficacy of soya intake on inflammatory biomarkers.
Acknowledgements
This paper was supported by the Student Research Committee, School of Nutrition and Food Science, Isfahan University of Medical Sciences.
This study was funded by Student Research Committee, Isfahan University of Medical Sciences (scientific code: 198193).
Contributions of the authors were: conceptualisation and formal analysis and methodology: M. R. and M. H. R.; data curation and investigation: M. R. and F. M.; project administration and supervision: M. H. R.; resources and writing - review and editing: M. H. R. and C. C. T. C.; visualisation: C. C. T. C.; software: M. R. and M. H. R. and F. M.; writing - original draft: M. R. and F. M. and C. C. T. C.
The authors declare that there are no conflicts of interest.