Barley (Hordeum vulgare L.) has been one of the most important grains for humans since ancient times, and it is the fourth most produced cereal in the world. The grain is mainly used for animal feed and beer production; however, during recent years, barley, especially the whole grain, has received an increased nutritional interest due to its positive effects on glucose and lipid metabolism( Reference Johansson, Nilsson and Östman 1 , Reference Ehrenbergerova, Brezinova Belcredi and Psota 2 ). Nutrient composition of barley varies widely among varieties due to genotype, environment and the interaction between these two factors( Reference Andersson, Lampi and Nystrom 3 ). Total dietary fibre in a typical whole-grain barley accounts for 15–24 g/100 g DM( Reference Andersson, Lampi and Nystrom 3 – Reference Nystrom, Lampi and Andersson 5 ), and the most interesting and abundant fibre components are β-glucans and arabinoxylans, known for their capacity to lower plasma cholesterol concentrations and improve postprandial glucose responses( Reference Garcia, Otto and Reich 6 , Reference Lu, Walker and Muir 7 ). Some barley grains also contain considerable amounts of resistant starch( Reference Bird, Flory and Davies 8 , Reference Shu, Backes and Rasmussen 9 ).
Health benefits of barley fibre may depend on its physico-chemical properties such as viscosity and water-holding capacity that affect cholesterol and bile acid excretion. Today, there is also an increasing interest in gut metabolites produced during colonic fermentation of dietary fibre and changes in the gut microbiota composition. The microbial end products from dietary fibre are SCFA (mainly acetic, propionic and butyric acids), of which butyric acid, in particular, plays an important role in promoting and maintaining colonic health( Reference Leonel and Alvarez-Leite 10 ). During recent years, metabolic effects and, interestingly, cognitive functions have also been related to butyric acid( Reference Nilsson, Johansson and Ekstrom 11 , Reference Valvassori, Varela and Arent 12 ). In contrast, propionic acid is generally associated with hypocholesterolaemic effects observed with the supplementation of soluble dietary fibre and different types of glucans (resistant starch, dextrins and oat β-glucans)( Reference Chen, Anderson and Jennings 13 – Reference Kozmus, Moura and Serrao 16 ). Furthermore, SCFA have been found to play a role in the immune system, and many of the anti-inflammatory effects are linked with G-protein-coupled receptors (GPCR) for NEFA (GPCR41 and GPCR43) expressed in various cells and tissues such as the intestinal tract and adipose tissue( Reference Maslowski, Vieira and Ng 17 , Reference Le Poul, Loison and Struyf 18 ). Barley fibre may also have a potential effect as a prebiotic, and the insoluble fraction of barley fibre has been shown to selectively increase the abundance of the phylum Actinobacteria in in vitro studies( Reference Rudi, Zimonja and Aasen 19 ), while soluble and viscous barley β-glucans have been reported to induce a high proportion of Lactobacillus in the caecum of rats( Reference Snart, Bibiloni and Grayson 20 ).
The amount and profile of SCFA formed in the colon can be influenced by microbiota composition, availability of dietary fibres and their physico-chemical properties, such as monomeric composition, type of linkage between monomeric units, degree of polymerisation, and solubility. Lower solubility of fructo-oligosaccharides has been associated with lower fermentability and production of SCFA in studies on rats, and the profile of SCFA formed from fructo-oligosaccharides depended on the degree of polymerisation( Reference Nilsson and Nyman 21 ). Furthermore, arabinoxylans and β-glucans are generally associated with high yields of propionic acid by human intestinal bacteria, as shown in in vitro studies and summarised by Rose( Reference Rose 22 ). It has been suggested from human studies that resistant starch may promote butyric acid formation in the colon, as judged by increased faecal excretion( Reference Noakes, Clifton and Nestel 23 , Reference van Munster, Tangerman and Nagengast 24 ). Barley β-glucans have been shown to stimulate butyric acid formation in the caecum of rats, and barley has been shown to increase plasma butyric acid concentrations in healthy subjects( Reference Johansson, Nilsson and Östman 1 , Reference Berggren, Björck and Nyman 25 ).
Low-grade systemic inflammation (endotoxaemia) is associated with obesity, diabetes and insulin resistance, and may be triggered by an unbalanced gut microbiota. Lipopolysaccharides (LPS) in Gram-negative bacteria are responsible for toxicity, causing inflammation when entering into the blood from the gut lumen. A high fat (HF) intake has been shown to increase intestinal permeability( Reference Cani, Bibiloni and Knauf 26 ) and also the circulating level of LPS( Reference Cani, Amar and Iglesias 27 ). In ruminants, high amounts of fat in the diet have been shown to impair the fermentation of dietary fibre( Reference Jenkins 28 , Reference Pantoja, Firkins and Eastridge 29 ), and studies have recently demonstrated the attenuation of fermentation effects in rats fed a HF diet( Reference Goldsmith, Martin and Raggio 30 , Reference Charrier, Martin and McCutcheon 31 ). A number of studies have also demonstrated that intake of a HF diet alters gut microbiota composition, with a decrease in the total number of bacteria and Bacteroidetes and an increase in the number of Firmicutes and Proteobacteria, and switches the microbiota balance towards a greater abundance of Gram-negative bacteria( Reference de La Serre, Ellis and Lee 32 , Reference Hildebrandt, Hoffmann and Sherrill-Mix 33 ). However, less is known about SCFA and other gut metabolites formed with HF diet intake. Lipopolysaccharide-binding protein (LBP) binds to LPS, and has been suggested as an adequate marker of LPS in the circulation, since it has a longer half-life and displays less interference with other compounds in blood than LPS( Reference Sun, Yu and Ye 34 – Reference Novitsky 36 ). Another component of interest in this context is monocyte chemoattractant protein-1 (MCP-1), which has been shown to increase in the blood of diabetic mice( Reference Higa, Liu and Berry 37 ).
The aim of the present study was to investigate the interaction between dietary fibre and fat on gut metabolites formed during fermentation in the hindgut of rats. For this purpose, two whole-grain barley varieties, containing different amounts of dietary fibre and β-glucans, were included in low-fat (LF) or HF diets. Hindgut carboxylic acids (CA), caecal microbiota, inflammatory markers in the circulation, and portal and hepatic lipid profiles were determined. The two whole-grain barley varieties were tested at the same level of fibre or flour, and consequently two levels of fibre and three levels of β-glucans were tested. Microcrystalline cellulose (MCC), known to be more or less resistant to colonic fermentation and thus giving minor amounts of CA, was used as a control.
Materials and methods
Test materials
Raw materials used were two hulled barley varieties containing different amounts of β-glucans: SW (8·2 g/100 dry-weight basis (dwb)) and Hadm (4·2 g/100 g, dwb) (Lantmännen SW Seed AB). MCC (FMC BioPolymer) was used as a control. The whole barley grains were milled to a particle size smaller than 0·5 mm before inclusion in the diets (CT193 Cyclotec™; FOSS).
Diets, study design and animals
All diets contained a basal diet mixture (330 g/kg, dwb)( Reference Jakobsdottir, Xu and Molin 38 ), including casein as protein source, dl-methionine, maize oil, sucrose, vitamins, choline chloride and minerals (see online Supplementary Table S1). The two whole-grain barley varieties were tested at the same level of fibre or flour. This resulted in three diets containing 80 g dietary fibre/kg (dwb, SW and Hadm) or 50 g dietary fibre/kg (dwb, 50Hadm), while the amount of whole-grain barley flour in the diet was 299 g/kg (dwb, 50Hadm and SW) or 481 g/kg (dwb, Hadm). This design also meant that the rats were given three levels of dietary β-glucans: 25·0 g/kg (dwb, SW), 20·0 g/kg (dwb, Hadm) and 13·0 g/kg (dwb, 50Hadm), respectively. MCC (80 g/kg, dwb) was used as the control. All the test diets were studied at a LF content (50 g/kg, dwb) and a HF content (240 g/kg, dwb). The HF diets contained butter (180 g/kg, dwb), a source of saturated fat common in Scandinavian countries, and cholesterol (10 g/kg, dwb). The study design resulted in eight test diets. The composition of the test diets is shown in online Supplementary Table S1. The DM content of the test diets was adjusted with wheat starch (Cargill), which has been shown to be completely digested, and so does not contribute to the formation of any CA in the hindgut of rats( Reference Björck, Nyman and Pedersen 39 ).
Male Wistar rats (Taconic), with an initial weight of 110 (sem 1) g, were randomly divided into eight groups of seven rats. Each group of rats was housed in subgroups of three or four rats per cage at room temperature of 22°C with a 12 h day and 12 h night cycle. The duration of the experiment lasted for 25 d. The rats were weighed at the 1st, 6th, 11th, 18th and the final day. Daily food intake was restricted to 12 g dry weight/rat for the first 11 d, increased to 15 g dry weight/rat, before reaching 20 g dry weight/rat in the final week. Free access to water was provided during the experiment. Food residues were collected and weighed daily.
On the final day, the rats were anaesthetised by subcutaneous injection of a mixture (1:1:2) of Hypnorm (Division of Janssen-Cilag Limited, Janssen Pharmaceutica), Dormicum (F. Hoffman-La Roche AG) and sterile water at a dose of 0·15 ml/100 g body weight. Liver, caecal tissue and content, and content of the distal colon were collected. Portal blood was drawn by syringe from the liver portal vein and sampled into two tubes, one for plasma containing EDTA and one for serum. Plasma and serum samples were centrifuged for 15 min (2500 g ) and then stored at − 40°C until analysis of SCFA (serum), amino acids, NH3, cholesterol, TAG and inflammatory markers (plasma). Caecal tissue and content, liver and epididymal and retroperitoneal fat pads were weighed, and the pH of caecal content was measured. Caecal content was divided into the following parts: one part was placed in a sterile tube, immediately frozen in liquid N2 and stored at − 80°C for analysis of the microbiota; the remainder and faecal pellets in the distal colon were frozen and stored at − 40°C until analysis of CA. Liver tissue was frozen and stored at − 40°C and then freeze-dried and milled before the analysis of cholesterol and TAG concentrations. The animal experiment was approved by the Ethics Committee for Animal Studies at Lund University (M56-12).
Analytical methods
Dietary fibre in whole-grain barley flour and microcrystalline cellulose
The amount of soluble and insoluble fibre in barley was determined gravimetrically( Reference Asp, Johansson and Hallmer 40 ). Neutral sugars in the isolated dietary fibre residues from barley and also in MCC were analysed by GLC and uronic acids by spectrophotometry. The mixed-linkage β-glucans in barley and in the isolated soluble fibre residues were determined using a commercial kit (Megazyme). Resistant starch barley was determined according to the method introduced by Granfeldt et al. ( Reference Granfeldt, Drews and Björck 41 ). Amylose and amylopectin were analysed using a commercial kit (Megazyme).
Carboxylic acids
SCFA (acetic, propionic, butyric, valeric, iso-valeric and iso-butyric acids) plus succinic and lactic acids are referred to as CA. Minor acids, presented in Table 3 and in the text, include valeric, iso-valeric and iso-butyric acids. GLC was used to determine SCFA in caecal and colon content( Reference Zhao, Nyman and Jonsson 42 ), and in portal serum( Reference Zhao, Liu and Nyman 43 ) with the GC ChemStation software (Agilent Technologies, Inc.) and HPDB-FFAP 125-3237 column (J&W Scientific, Agilent Technologies, Inc.).
In brief, samples from the caecum and colon were acidified by 0·25 m-HCl and homogenised (IKA®-WERKE) with 2-ethylbutyric acid (Sigma-Aldrich Chemical Company) as the internal standard. The samples were then centrifuged and supernatants injected directly onto the fused-silica capillary column. SCFA in portal serum was protonated with 2 m-HCl, and then concentrated on a shaker (VXR; IKA®-Labortechnik) for 16 h at 350 rpm using hollow fibre-supported liquid membrane extraction with tri-n-octylphosphine oxide in dihexyl ether as the liquid membrane and 0·1 m-NaCl as the acceptor.
Succinic acid and lactic acid in the caecum were determined by ion-exclusion chromatography, with a method developed in our laboratory( Reference Liu and Wang 44 ). Samples from caecal content prepared for SCFA analysis were further diluted with Millipore water, filtered through a 0·45 mm syringe filter, and injected onto a Metrosep organic column (250 × 7·8 mm; Metrohm), with the column oven set at 70°C. The flow rate of eluent (0·5 mm-H2SO4) was 0·6 ml/min. Each run of the sample lasted for 25 min. The suppressor was regenerated with 10 mm-LiCl solution followed by Millipore water.
Amino acids and ammonia
Free amino acids (n 18) and NH3 in portal plasma were analysed with an amino acid analyser (Biochrom 30; Biochrom Limited) based on ion-exchange chromatography. A precipitation step was used to purify the sample( Reference Stenberg, Marko-Varga and Öste 45 ), and N-isobutylglycine was used as the internal standard. The EZChrom Elite software package (Scientific Software, Inc.) was applied to evaluate the results.
Quantitative PCR
Abundances of total bacteria, Lactobacillus, Bifidobacterium, Akkermansia, Clostridium leptum group and Bacteroides fragilis group in the caecum of rats were quantified by real-time quantitative PCR using Bio-Rad real-time PCR system and software. Primer sequences are listed in online Supplementary Table S2. Real-time quantitative PCR was performed in a reaction volume of 25 μl, with 10 μl of SsoAdvanced SYBR Supermix (Bio-Rad), 300 nm each of the forward and reverse primers, and 1 μl of caecal DNA samples (EZ1 DNA Tissue Kit and BioRobot EZ1 workstation; Qiagen AB). The PCR conditions were 95°C for 3 min, followed by forty cycles at 95°C for 10 s and annealing temperature for 30 s. A melt curve analysis was performed after amplification for quality assurance and specificity of the PCR. Standards were kindly provided by Dr Xu( Reference Xu 46 ).
Inflammatory marker assays
IL-4, IL-18, TNF-α and MCP-1 in the portal plasma were analysed in duplicate using a Rat Cytokine/Chemokine Magnetic Bead Panel kit (Millipore Corporation), and LBP in the portal plasma was determined by the LBP ELISA Kit for a wide variety of species (Hycult Biotech), according to the manufacturer's instructions.
Cholesterol and TAG
Lipids in the liver were extracted by a modified protocol( Reference Hara and Radin 47 ). Duplicates of 20 mg freeze-dried samples were extracted with hexane–isopropanol (3:2, v/v) with the addition of 0·005 % 2,6-di-tert-butyl-4-methylphenol. The extract was redissolved in 1 ml isopropanol+Triton X-100 1 % after being dried under N2. The solution was used to determine total cholesterol and TAG concentrations by Infinity cholesterol/TAG Liquid Stable reagent (Thermo Trace). Cholesterol and TAG levels in the portal plasma were determined using the same assay as used for the liver.
Calculations and statistical analyses
Caecal pools of CA (μmol) were obtained by multiplying the concentration of each CA (μmol/g) with caecal content. Body-weight gain was presented either as a percentage increase compared with the initial body weight or increase per g consumed feed.
Two-way ANOVA with Tukey's test was applied to evaluate the effects of dietary fibre (including the four variables: control containing MCC, the two varieties SW and Hadm (80 g/kg), and the lower level of Hadm in the diet (50 g/kg)), fat (LF and HF) and their interactions. The effects of dietary fibre and fat were only considered significant when the corresponding P value was less than 0·05 and no interaction was present (P>0·1). If the main effects of dietary fibre were significant, the means of the LF and HF groups consuming the same fibre were compared using Tukey's test (P< 0·05). When the interaction effect was present, each individual group was evaluated with one-way ANOVA followed by Tukey's test (P< 0·05). Before statistical evaluation, non-normally distributed data were transformed with Box–Cox transformation. If the data were still abnormally distributed after transformation, the Kruskal–Wallis test was applied instead, followed by the Nemenyi–Damico–Wolfe–Dunn test using the package coin in the statistical software R2.15.2 (R Foundation for Statistical Computing). MINITAB statistical software (release 16; Minitab Inc.) was used for the univariate statistical evaluation. Tendency was defined as 0·05 < P <0·1.
Since the experiment included many test variables, results were also subjected to multivariate data analysis by means of principal component analysis using SIMCA 13.0 (Umetrics) to show differences between the groups.
Results
Dietary fibre in raw materials
Dietary fibre content was higher in the barley variety SW than in Hadm (26·7 g/100 g, dwb v. 16·7 g/100 g, dwb; Table 1); of the total fibre content, 31 and 26 %, respectively, were β-glucans, leading to a lower proportion of non-β-glucans (probably cellulose) in SW (4 %) than in Hadm (13 %). The proportion of xylose and arabinose of total dietary fibre polysaccharides was similar in the two barley varieties, i.e. 24–27 and 13–14 %, respectively. Mannose, galactose and uronic acids were also detected (2–4 %). Only minor amounts of resistant starch were found in the two varieties (0·3 g/100 g dwb in SW and 0·8 g/100 g dwb in Hadm). Starch consisted of 23·5 and 25 % amylose in SW and Hadm, respectively (data not shown). MCC consisted of 94 % glucose and 3 % each of xylose and mannose.
Table 1 Dietary fibre content (g/100 g, dry-weight basis), soluble fraction (%) and composition (% of dietary fibre polysaccharides) in microcrystalline cellulose (MCC, FMC BioPolymer; control), Hadm and SW barley (Mean values with their standard errors or percentages, n 2)
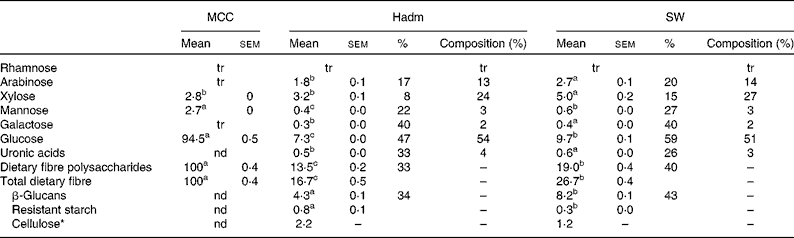
tr, Trace ( < 0·05 g/100 g); nd, not determined.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* Cellulose was obtained by subtracting the values of β-glucans and resistant starch from those of glucose.
Feed intake, weight gain, caecal content, pH and tissue weight
All rats were active and gained body weight throughout the experiment. Daily food intake per rat averaged between 13·8 and 14·9 g, and the rats had gained between 114 and 172 g by the final day, which corresponded to 106 and 162 %, respectively, compared with the initial body weight (Table 2).
Table 2 Feed intake (g/rat per d), body-weight gain per initial body weight (%), body-weight gain per feed (g/g feed), caecal content (g), tissue weight (g) and pH, spleen weight (g), fresh and dry liver weight (g), and epididymal and retroperitoneal fat weight (g) in rats given two barley varieties (Hadm and SW) or a control diet at a low and high fat content for 25 d (Mean values with their standard errors, n 7)

a,b,cMean values within a row with unlike superscript letters were significantly different.
* Values for groups fed the barley diets were significantly higher than those fed the control diet.
† Values for groups fed the barley diets were significantly higher than those fed the control diet, and values for groups fed the SW diet were significantly higher than those fed the 50Hadm diet.
‡ Values for groups fed the barley diets were significantly lower than those fed the control diet.
§ Total dietary fibre content in the diet.
∥ β-Glucan content in the diet.
Barley diets gave a higher body-weight gain (as a percentage of the initial body weight), caecal content, caecal tissue weight and liver weight than the control (P< 0·05), while caecal pH was lower (P< 0·05) (Table 2). Furthermore, rats fed SW had a higher caecal content than those fed 50Hadm (P< 0·05). Weights of the spleen, epididymal and retroperitoneal fat pads were similar between the barley groups and the control.
Rats fed the HF diets had a higher body-weight gain (as a percentage of the initial body weight) than those fed the LF diets (P< 0·001; Table 2). This could also be observed when calculated in g/g feed, and the increase was between 0·30 and 0·38 for rats fed the LF diets and between 0·38 and 0·47 for those fed the HF diets. Weights of the caecal content, liver, spleen and epididymal fat pads were also higher in rats fed the HF diets than in those fed the LF diets (P< 0·05), while caecal pH was lower (P< 0·01). The effects of fat on epididymal and retroperitoneal fat pads were different between the groups fed the barley diets and the control diet. Thus, the weight of fat pads generally increased more in rats fed the barley diets (mean 33 and 61 % for epididymal and retroperitoneal fat pads, respectively) than in those fed the control diet (11 and 26 %, respectively).
Carboxylic acid
The relative mean distributions of the major SCFA (acetic, propionic and butyric acids) in the caecum were 75, 9 and 16 % in the LF groups v. 81, 12 and 7 % in the HF groups. The corresponding values in the distal colon were 79, 10 and 11 % in the LF groups and 79, 14 and 7 % in the HF groups, and those in the portal blood were 84, 6 and 10 % in the LF groups and 87, 8 and 5 % in the HF groups (data not shown).
Caecum
The total caecal pool (μmol) of SCFA was higher in rats fed the barley diets than the control diet (P< 0·001; Table 3). This was also reflected for the specific SCFA, except the minor ones in rats fed HF diets. Furthermore, rats fed the higher fibre level of barley had higher amounts of total SCFA and acetic acid than those fed 50Hadm (P< 0·05). The caecal pool of propionic acid was also higher in rats fed SW than in those fed 50Hadm (P< 0·05). Concerning butyric acid, the amount was higher, but unaffected by fibre and β-glucan content in rats fed the barley diets compared with those fed the LF control diets. In the HF setting, the amount of butyric acid was higher in rats fed diets containing the higher content of dietary fibre (SW and Hadm) than the control diet (P< 0·01). Furthermore, the amount of butyric acid was higher in rats fed the highest amount of β-glucans (SW) than in those fed the lowest amount (50Hadm) (P< 0·01).
Table 3 Carboxylic acids in the caecum (μmol or μmol/g), distal colon (μmol/g) and portal serum (μmol/l) of rats given two barley varieties (Hadm and SW) or a control diet at a low and high fat content for 25 d (Mean values with their standard errors; n 7 except for high fat control portal serum samples (n 6))

a,b,cMean values within a row with unlike superscript letters were significantly different.
* Values for groups fed the barley diets were significantly higher than those fed the control diet, and those fed the SW and Hadm diets had significantly higher values than those fed the 50Hadm diet.
† Values for groups fed the barley diets were significantly higher than those fed the control diet, and values for groups fed the SW diet were significantly higher than those fed the 50Hadm diet.
‡ Values for groups fed the barley diets were significantly higher than those fed the control diet.
§ Total dietary fibre content in the diet.
∥ β-Glucan content in the diet.
The caecal pool of SCFA in rats fed the HF and barley diets was higher than that in rats given the corresponding LF diets, while caecal pools in rats fed the LF and HF controls were very similar (Table 3). The higher values with barley were generally reflected in the specific SCFA. Exceptions were butyric acid and the minor acids, which were generally lower with HF barley diets (although not always significant), while corresponding values with the control diet were very similar. The difference between the LF and HF diets with respect to the amount of butyric acid formed in rats also seemed to decrease with an increasing level of β-glucans in the diet (SW). HF content also increased the caecal pool of lactic acid in rats fed the barley diets (P< 0·05). No effect of HF content on lactic acid could be observed in rats fed the MCC (control) diet. Furthermore, HF content in the diets increased the amount of succinic acid by over twenty times in rats fed the barley diets, while there was only a moderate increase in the control group (three times higher, P< 0·05; Mann–Whitney test).
The concentration (μmol/g) of total SCFA in the caecum was higher in rats fed the barley diets than in those fed the MCC (control) diet (P< 0·01; Table 3). Fat had no effect on SCFA concentration.
Distal colon
As in the caecum, rats fed the barley diets had higher concentrations (μmol/g) of total SCFA than those fed the control diet (P< 0·001), mainly due to increased concentrations of acetic acid (P< 0·001) (Table 3). Furthermore, the concentration of propionic acid was higher in rats fed SW (P< 0·001) and Hadm (P< 0·05) compared with those fed the control diet.
The HF content of the diet increased the concentration of acetic acid in rats compared with the LF content (P< 0·01). The decrease in butyric acid concentration was significant (P< 0·01) between the LF and HF diets in rats fed the SW and 50Hadm diets. No effect of HF content was observed in rats fed the control diet.
Portal blood
Serum concentrations (μmol/l) of total and specific SCFA were generally higher in rats fed the barley diets than in those fed the control diet (P< 0·001). There were no effects of variety or level of dietary fibre (Table 3).
The HF diets containing barley generally lowered the serum concentrations of butyric acid and the minor SCFA (Table 3). The serum concentration of propionic acid was higher in rats fed the HF 50Hadm diet than in those fed the LF counterpart.
Amino acids in portal blood
The major amino acids in portal plasma were glutamine, alanine, lysine, threonine and glycine (Table 4). The concentrations (μmol/l) of most amino acids in the portal plasma of rats were similar in rats fed the barley and control diets, but there were some exceptions. Barley groups exhibited lower concentrations of serine and glycine (only for 50Hadm and SW) compared with the control group (P< 0·001), whereas concentrations of leucine and arginine were higher in rats fed Hadm (P< 0·05). Furthermore, tyrosine concentration was higher in rats fed Hadm than in those fed SW (P< 0·05).
Table 4 Concentrations of free amino acids and ammonia in the portal plasma (μmol/l) in rats fed two barley varieties (Hadm and SW) or a control diet at a low and high fat content for 25 d (Mean values with their standard errors; n 7 except for control (high and low fat) portal plasma samples (n 6))

a,b,cMean values within a row with unlike superscript letters were significantly different.
* Values for groups fed the Hadm diet were significantly higher than those fed the control diet.
† Values for groups fed the 50Hadm and SW diets were significantly lower than those fed the control diet.
‡ Values for groups fed the barley diets were significantly lower than those fed the control diet.
§ Values for groups fed the Hadm diet were significantly higher than those fed the SW diet.
∥ Total dietary fibre content in the diet.
¶ β-Glucan content in the diet.
The portal concentration of total amino acids was higher in the HF groups than in the LF groups (P< 0·001), a fact that could be observed with most specific amino acids. Exceptions were methionine, cysteine, isoleucine and phenylalanine (not shown in Table 4), where similar concentrations were detected for both HF and LF diets. Furthermore, threonine concentration was higher in rats fed the HF barley diets than in those fed the HF control diet (P< 0·01). The values of threonine in the HF control group were also lower than those in the LF group (P< 0·01).
No difference was observed in NH3 between the groups, neither by adding barley or fat.
Quantitative PCR of caecal microbiota
There was an increased abundance of Lactobacillus (P< 0·05) in rats fed the barley diets at the higher level of dietary fibre (80 g/kg) and a decrease in the number of B. fragilis (P< 0·01) in rats fed the two barley diets containing Hadm, compared with the control (Fig. 1(A)). The abundance of Bifidobacterium was also higher in rats fed the LF barley diets than in those fed the control diet (P< 0·01), whereas the abundance of Akkermansia was lower (seventy times lower for SW, 1000 times for Hadm and 3000 times for 50Hadm).

Fig. 1 Effects of two barley varieties and dietary fibre levels on (A) caecal microbiota, (B) lipids in blood and liver, and (C) plasma lipopolysaccharide-binding protein (LBP) and monocyte chemoattractant protein-1 (MCP-1) in rats fed low-fat (![]() ) and high-fat (
) and high-fat (![]() ) diets. n 7 rats per group, except n 6 for (B, C) control high-fat samples (plasma) and (C) SW high fat samples. Values are means, with their standard errors represented by vertical bars. Mean values with unlike letters were significantly different (P< 0·05; one-way ANOVA or Kruskal–Wallis). Total bacteria, P
fibre>0·1, P
fat< 0·001; Lactobacillus, P
fibre= 0·016, P
fat< 0·001; Bacteroides fragilis group, P
fibre= 0·002, P
fat>0·1; plasma cholesterol, P
fibre>0·1, P
fat< 0·001; plasma TAG, P
fibre>0·1, P
fat= 0·099; LBP, P
fibre< 0·001, P
fat< 0·001; MCP-1, P
fibre= 0·03, P
fat>0·1. C. leptum, Clostridium leptum.
) diets. n 7 rats per group, except n 6 for (B, C) control high-fat samples (plasma) and (C) SW high fat samples. Values are means, with their standard errors represented by vertical bars. Mean values with unlike letters were significantly different (P< 0·05; one-way ANOVA or Kruskal–Wallis). Total bacteria, P
fibre>0·1, P
fat< 0·001; Lactobacillus, P
fibre= 0·016, P
fat< 0·001; Bacteroides fragilis group, P
fibre= 0·002, P
fat>0·1; plasma cholesterol, P
fibre>0·1, P
fat< 0·001; plasma TAG, P
fibre>0·1, P
fat= 0·099; LBP, P
fibre< 0·001, P
fat< 0·001; MCP-1, P
fibre= 0·03, P
fat>0·1. C. leptum, Clostridium leptum.
In rats fed the HF diets, populations of total bacteria and Lactobacillus were lower compared with the LF diets (P< 0·001) (Fig. 1(A)). Furthermore, there was a reduction in the population of Bifidobacterium in rats fed Hadm (P< 0·001), as well as a reduction in the C. leptum group population in rats fed Hadm and 50Hadm (P< 0·001). The abundance of Akkermansia was much higher in rats fed the HF barley diets than in those fed the LF barley diets (500, 10 000 and 40 000 times higher for SW, Hadm and 50Hadm, respectively), whereas the HF diet-induced increase (P= 0·0553; Mann–Whitney) in rats fed the control diets was much less (four times higher).
Cholesterol and TAG in plasma and liver
The HF diets generally induced a higher amount of lipids in the plasma and liver (Fig. 1(B)). Only the highest level of β-glucan content (SW) protected against the HF-induced increase in the concentration of plasma cholesterol (5·1 (sem 0·2) mmol/l for the LF diet and 5·2 (sem 0·2) mmol/l for the HF diet). The rats fed barley had a higher amount of liver TAG concentrations than those fed the control diet (P< 0·001). The level of portal plasma TAG tended to be higher in rats fed the HF barley diets than those fed the LF barley diets (P= 0·099).
Inflammatory markers
The levels of plasma LBP and MCP-1 were lower in rats fed barley than in those fed the control diet (P< 0·05; Fig. 1(C)). Furthermore, the level of plasma LBP was higher in rats fed the HF diets than in those fed the LF diets (P< 0·05). The level of MCP-1 was not determined in samples from rats fed 50Hadm due to instrument failure. The levels of IL-4, IL-18, IFN-γ and TNF-α in the portal plasma were below the detection limit with the method used.
Multivariate analysis
The bi-plot based on the principal component analysis reveals an arrangement of test groups based on the measured parameters, and summarises the relationship between the groups and parameters by their locations in the plot. Only the relevant variables are shown in Fig. 2. A clear grouping of data was observed in the plot, and rats fed the LF control, HF control, LF barley and HF barley diets were clearly distinguished from each other. Each dot in the plot represents eighty-one analyses. The caecal pool of butyric acid, the caecal abundance of Lactobacillus, Bifidobacterium and the C. leptum group, and plasma threonine concentration were high in the LF barley groups, while total SCFA, acetic acid, propionic acid and succinic acid concentrations were high in the HF barley groups. Caecal pH was high in the LF control groups, and plasma MCP-1 level was high in the HF control groups. A positive correlation was observed between the caecal abundance of Akkermansia and plasma LBP level.

Fig. 2 Bi-plot of principal component analysis. The location of each triangle (![]() , low-fat control (LF-control);
, low-fat control (LF-control); ![]() , LF-50Hadm;
, LF-50Hadm; ![]() , LF-Hadm;
, LF-Hadm; ![]() , LF-SW) and circle (
, LF-SW) and circle (![]() , high-fat control (HF-control);
, high-fat control (HF-control); ![]() , HF-50Hadm;
, HF-50Hadm; ![]() , HF-Hadm;
, HF-Hadm; ![]() , HF-SW) is based on the collection of all investigated parameters (n 81) in each rat.
, HF-SW) is based on the collection of all investigated parameters (n 81) in each rat. ![]() represents experimental parameters analysed in the present study, and for graphical presentation, only selected ones are shown. Red-green frame, control; green frame, barley-LF; red frame, barley-HF.
represents experimental parameters analysed in the present study, and for graphical presentation, only selected ones are shown. Red-green frame, control; green frame, barley-LF; red frame, barley-HF. ![]() indicates the classification of rats fed the LF or HF diets. C. leptum, Clostridium leptum; B. fragilis, Bacteroides fragilis; LBP, lipopolysaccharide-binding protein; MCP-1, monocyte chemoattractant protein-1. R
2
x(1) = 0·342; R
2
x(2) = 0·179.
indicates the classification of rats fed the LF or HF diets. C. leptum, Clostridium leptum; B. fragilis, Bacteroides fragilis; LBP, lipopolysaccharide-binding protein; MCP-1, monocyte chemoattractant protein-1. R
2
x(1) = 0·342; R
2
x(2) = 0·179.
Discussion
Insulin resistance and related diseases and symptoms are associated with elevated low-grade systemic inflammation, which may be triggered by a HF diet and an unbalanced microbiota. In this context, it is important to examine the role and interaction of dietary factors. Barley has increasingly been associated with nutritional effects, mainly due to its content of β-glucans( Reference Borneo and Leon 48 ), and the variation is large between different varieties. We selected two whole-grain barley varieties, SW and Hadm, differing in the content of dietary fibre and β-glucans and also in the solubility of dietary fibre. The design of the present study resulted in two levels of dietary fibre and three levels of β-glucans. We investigated caecal SCFA, microbial composition, inflammatory markers in the circulation, and portal and hepatic lipid profile in rats fed LF and HF diets.
Intake of SW and Hadm at the same dietary fibre level (80 g/kg, dwb) produced similar amounts and profiles of SCFA formed in the caecum of rats, while fewer amounts of SCFA were formed in rats fed 50Hadm, as expected. Thus, the higher fraction of soluble fibre in SW did not increase the amount of caecal SCFA, which might have been expected, perhaps because the difference in fibre solubility between the two barley varieties was too small. Resistant starch is present in large amounts in some types of barley( Reference Bird, Flory and Davies 8 ); however, the barley varieties that we used contained very limited amounts of resistant starch, thus contributing only with minor amounts of SCFA. Rats consuming the barley diets also showed a similar behaviour in terms of body-weight gain, caecal pH and caecal tissue weight, which not would have been the case if any of the barley varieties had contained more resistant starch than the other one.
The barley diet containing the highest amount of β-glucans and a higher fraction of soluble β-glucans (SW) had a hypocholesterolaemic effect, while no such effect could be observed with the other barley diets. This is interesting, since barley and barley β-glucans, as well as oat and oat β-glucans, have well-documented effects regarding their cholesterol-lowering capacities in healthy subjects( Reference Wolever, Tosh and Gibbs 49 ). Intake of at least 3 g β-glucan per d is recommended by the European Food Safety Authority( 50 ). While it is still uncertain whether the effect is dose-dependent( Reference Tiwari and Cummins 51 , Reference AbuMweis, Jew and Ames 52 ), no effect at a dose of 3·3 g/d has been reported in healthy subjects( Reference Ibrugger, Kristensen and Poulsen 53 ). It has been pointed out that factors such as solubility and viscosity of dietary fibre might be crucial for the effect( Reference Ibrugger, Kristensen and Poulsen 53 ). The results from the present study suggest that a higher intake (25 g β-glucans per kg feed) is needed to achieve hypocholesterolaemic effects in rats fed the HF diet. Except for solubility and viscosity of β-glucans, gut metabolites formed during fermentation have been suggested to have an effect. Propionic acid has been proposed to play a role in this context, which has been observed in rats receiving oral administration of propionic acid or diets containing high amounts of propionic acid such as oats( Reference Chen, Anderson and Jennings 13 , Reference Immerstrand, Andersson and Wange 15 ), but this is still in debate. However, we could validate higher caecal pools of propionic acid in rats fed barley, especially in those fed SW than in those fed the control diet, and it might be speculated whether this is due to the higher content of β-glucans and/or solubility of dietary fibre. Furthermore, SW with the highest amount of β-glucans was the barley diet that counteracted the decreased formation of butyric acid in rats fed the HF diets to the highest extent.
Feeding the HF diets had a considerable impact on the biomarkers of interest. The caecal amount of butyric acid was considerably lower when shifting the diet from a LF to a HF content; for example, the amount of butyric acid was reduced by 27 % in the SW group and 53 % in the Hadm group. At the lowest level of β-glucans (50Hadm), the reduction was even higher (73 %). Thus, it seems as if the level of β-glucans, or their effects on the microbiota composition, counteracted this reduction. A similar trend was observed in the portal blood. However, it should be noted that the dose effects of β-glucans and dietary fibre in the diet were generally present in HF-fed rats, while in the LF setting, dose effects on SCFA profiles were not that apparent, such as the effect on butyric acid. Butyric acid has been shown to induce differentiation of regulatory T-cells in the colon and to have anti-inflammatory properties( Reference Furusawa, Obata and Fukuda 54 ), and the present study suggests that an increased amount of β-glucans is required when consuming a fat-rich diet to maintain the amount of butyric acid formed. In the control groups, which contained a fibre resistant to fermentation, low and similar amounts of butyric acid were formed in rats fed the LF and HF diets. Interestingly, we observed a negative correlation between butyric acid concentration in the caecum and portal serum and plasma LBP levels (r − 0·418, P= 0·002 for caecum content and r − 0·343, P= 0·013 for portal serum). Furthermore, all the barley diets prevented the HF diet-induced increase in LBP levels, indicating that intake of barley per se decreased the levels of LBP, irrespective of the fibre or β-glucan level. In addition to the effect on the levels of LBP, we also found that whole-grain barley could lower the plasma level of MCP-1 in rats fed the HF diets.
Another bacterial metabolite produced with HF diets and barley, but not with the control diet, was succinic acid. This is an intermediary microbial product, usually found at low bacterial activity, such as during antibiotic treatment( Reference Berggren 55 ). In contrast, resistant protein has been reported to lower the high amount of succinic acid induced by a high amount of dietary fibre( Reference Morita, Kasaoka and Hase 56 ); however, we do not know whether any protein was delivered to the hindgut in the present study (see below). The role of succinic acid in the gut is still unclear, and also its tolerance level; however, it has been suggested that treatment that would give rise to high production of succinic acid should be avoided for patients with ulcerative colitis, due to its inhibitory effect on the proliferation of epithelial cells, as well as its reducing effect on the crypt size in the colon( Reference Inagaki, Ichikawa and Sakata 57 ). A correlation between caecal concentration of succinic acid and caecal inflammation has been established in a colitis model( Reference Setoyama, Imaoka and Ishikawa 58 ). More studies are needed to give a better explanation and to predict the effect of the enrichment of succinic acid by HF intake together with fermentable dietary fibres such as barley. It may be questioned whether the high presence of caecal succinic acid can be related to the reduction in the abundance of the C. leptum group in the rats, which is an important group of butyric acid producers that includes Faecalibacterium prausnitzii and certain species of Eubacterium and Ruminococcus. There was a correlation with the decreased amount of butyric acid and the decreased abundance of the C. leptum group (r 0·629, P< 0·001). There was also a decreased abundance of total bacteria and Lactobacillus in rats fed HF barley compared with those fed LF barley in the present study.
A higher abundance of Akkermansia was also induced by the HF diet, particularly in the barley groups. Akkermansia is a commensal resident in the human gut, with Akkermansia muciniphila as the only species that has so far been discovered in this genus( Reference Derrien, Vaughan and Plugge 59 ). A. muciniphila is known to utilise mucin, the skeleton of the mucus layer, and to produce acetic and propionic acids as fuel for colonocytes( Reference Derrien, Vaughan and Plugge 59 ). This could be a plausible explanation for the increased caecal pool of those acids, at least for propionic acid, in the HF group as shown in the present study, and we found that abundance was positively correlated with caecal amount of propionic acid (r 0·357, P< 0·001) and interestingly also with the amount of succinic acid (r 0·487, P< 0·001). A. muciniphila has been suggested as a candidate probiotic( Reference Ferreira, Leonel and Melo 60 ), and a reversed HF-induced metabolic disorder by this species has been demonstrated( Reference Everard, Belzer and Geurts 61 ). However, compared with the much higher abundance in the HF barley groups, the abundance of Akkermansia was quite low in the LF counterparts in the present study, which suggests a need to further evaluate the probiotic role of Akkermansia. Our previous study showed similar results concerning acetic, propionic and succinic acids and Akkermansia, with pectin and guar gum( Reference Jakobsdottir, Xu and Molin 38 ).
Another interesting finding was the increased concentration of plasma amino acids induced by HF diets, which was independent of fibre source, indicating that fermentation and bacterial metabolism had minor effects. Furthermore, a study( Reference Do, Hindlet and Waligora-Dupriet 62 ) has reported an increase in the plasma concentration of amino acids after HF feeding in mice, for which several explanations were suggested by the authors. These included a specific and adaptive absorption of amino acids, induction of specific amino acid transporters in the small intestine and colon, modulation in intestinal permeability or motility, and modulation in the gut microbiota( Reference Do, Hindlet and Waligora-Dupriet 62 ). So far, we do not know the consequence of the increased concentration of amino acids stimulated by fat; however, lipids together with branched-chain amino acids may synergise to promote metabolic diseases( Reference Newgard Christopher 63 ). Apart from amino acids whose concentrations were increased, threonine, an essential amino acid, was lower in rats fed the HF diets than the LF diets; however, it was recovered by supplementing with barley. It is still unclear why threonine behaved differently from other amino acids; however, as a core component of the mucus layer, deficiency of threonine might be associated with the abnormal mucus layer induced by fat( Reference Everard, Belzer and Geurts 61 , Reference Waldén, Ahl and Jägare 64 ). Interestingly, a negative correlation was shown between threonine and the caecal abundance of Akkermansia (r − 0·294, P= 0·031), a mucin utiliser.
Conclusion
In conclusion, supplementing LF or HF diets with whole-grain barley increased the amount of total SCFA in the caecum of rats. Barley together with HF content led to a higher caecal pool of acetic acid and propionic acid, whereas HF content decreased the caecal amount of butyric acid. However, the caecal amount was recovered to a great extent with the highest level of β-glucans. Intake of barley reduced inflammation and altered the gut microbiota, as shown by the lowered LBP and MCP-1 levels in the circulation and the higher abundance of Lactobacillus and Bifidobacterium, together with the lower abundance of the B. fragilis group in the caecum. HF diets gave rise to a higher concentration of the majority of amino acids and a lower concentration of threonine in the portal blood of rats. However, the lowered concentration of threonine could be normalised by the consumption of barley. No improvement on lipids in the liver, fat tissue and body weight was observed in rats fed either of the barley varieties, and the high abundance of Akkermansia and succinic acid in the caecum stimulated by the consumption of HF with barley needs further investigation. Finally, suppression of HF diet-induced hypercholestolaemia was observed by the intake of the highest level of β-glucans. The interaction between fat and fibre in the diet influenced the effects in rats, where dose effects of dietary fibre and β-glucans were most apparent in the HF setting.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515000793
Acknowledgements
The authors thank Dr Ulf Nilsson and Dr Greta Jakobsdottir for their technical assistance with the animal experiments, and Dr Jie Xu for providing standards for the bacterial assay.
The present study was funded by the Albert Påhlsson Foundation. Y. Z. was financially supported by the Chinese Scholarship Council. The authors also thank Lantmännen SW Seed AB for kindly providing the whole-grain barley material and Skånemejerier for providing the butter.
The authors' contributions are as followers: Y. Z. and M. N. designed the study; Y. Z. performed the study, evaluated the data statistically and was responsible for the writing of manuscript; N. M. conducted the analysis of LBP and Bifidobacterium; M. N. and F. F. participated in the writing, reviewing and revising of the manuscript.
The authors declare that they have no conflicts of interest.











