-
PDF
- Split View
-
Views
-
Cite
Cite
Anthony Wilson, Duncan Simpson, Elizabeth Chandler, Phil Jennings, Paul Nicholson, Development of PCR assays for the detection and differentiation of Fusarium sporotrichioides and Fusarium langsethiae, FEMS Microbiology Letters, Volume 233, Issue 1, April 2004, Pages 69–76, https://doi.org/10.1016/j.femsle.2004.01.040
Close - Share Icon Share
Abstract
Isolates of the type-A trichothecene producing Fusarium sporotrichioides and Fusarium langsethiae were grouped and differentiated in a phylogenetic tree using ITS sequence dissimilarity. An attempt was made to develop a PCR-based assay for the detection and differentiation of Fusarium sporotrichiodes from other Fusarium species using the 5′-region of the tri5 gene as a template. However, this assay was unable to differentiate, to a satisfactory level, between isolates of Fusarium sporotrichioides and Fusarium langsethiae, providing further genetic evidence for their close genetic relationship. A robust and repeatable PCR-assay was developed for the detection and differentiation of both species based on sequence determined from differentially amplified RAPD-PCR products. These assays were able to detect both species in samples of grain taken from the field.
1 Introduction
Fusarium head blight (FHB), also known as scab or fusarium ear blight (FEB), is caused by a number of species, predominantly Fusarium culmorum, F. graminearum, F. poae, F. avenaceum and Microdochium nivale (varieties majus and nivale) [1]. These species also cause brown foot rot and seedling blight. Other species of Fusarium, such as F. avenaceum and F. poae, although generally less damaging, are also associated with head blight. In other recent studies, common species in the temperate regions of Northwest Europe included F. sporotrichioides [2, 3] and F. equiseti[3].
FHB can significantly reduce grain yield and quality [4]. Affected ears produce shrivelled or ‘tombstone’ kernels, which do not have the requisite weight to be successfully harvested. Moreover, FHB causes indirect loss by reducing seed germination, and causing blight of seedlings and poor stand establishment [5]. Depending on the species of Fusarium causing the disease, the wheat genotype and the environmental conditions, yield loss estimates vary from 6.4–70%[1].
Fusarium species produce mycotoxins [6, 7]. F. equiseti, F. graminearum, F. culmorum, F. poae and F. sporotrichioides are considered the most toxic Fusarium species in animal feed stuffs and the increasing incidence of mycotoxins in grain, along with the highly toxic nature of these mycotoxins [8, 6] are of particular concern to inter-governmental trade organisations such as the European Union [9]. Trichothecene mycotoxins produced by Fusarium species have been subdivided into type-A trichothecenes, which include diacetoxyscirpenol (DAS), T-2 and HT-2, and type-B trichothecenes such as deoxynivalenol (DON) and nivalenol (NIV). This categorisation is based upon the absence (type-A) or presence (type-B) of a keto group at C-8 of the trichothecene skeleton. F. sporotrichioides, F. poae and F. equiseti are type-A producers, whereas F. graminearum and F. culmorum produce type-B trichothecenes. T-2 toxin is cytotoxic [10], inhibits protein synthesis and reduces lymphocyte proliferation [11]. T-2 is regarded as the most toxic trichothecene produced by Fusarium spp. [12].
The need to develop rapid, inexpensive and reliable methodologies of identification is necessitated by the disease being a complex of species, and by the potential of different members of the genus Fusarium to produce different mycotoxins [8, 13, 6]. PCR-based assays have been reported for type-B trichothecene producers F. culmorum [14, 15], F. graminearum [14, 15], F. pseudograminearum[16] and F. cerealis[17]. Few assays for the detection of type-A producers, important on cereals, other than F. poae[18] have been reported, further highlighting the need for appropriate assays. Accurate identification in the field of the type-A (T-2, HT-2) producer F. sporotrichioides is imperative in order to determine the potential risk to human and animal health of individual FHB outbreaks.
2 Materials and methods
2.1 Origin and maintenance of fungal isolates
Isolates of F. sporotrichioides and other Fusarium species used in this study, along with their sources, are shown in Table 1. The isolates of Fusarium and other fungi used in this study are held in the John Innes Centre ‘facultative pathogen’ fungal collection. For long-term preservation, cultures were stored in vapour-phase liquid nitrogen vessels. For use in the present study, cultures were maintained at 15 °C on potato dextrose agar (PDA) (Difco) in 9 cm Petri-dishes.
Code, origin of isolates, species name and amplification through PCR using tri5 primers (Spo3F and Spo1R), F. sporotrichioides specific primers (Fspo and LanspoR1), F. langsethiae specific primers (FlangF3 and LanspoR1) and primers to both species (127F1/127R2) of Fusarium and Microdochium species used during this study
| Species | Code | Country | spo3F/spo1R | fspo/lanspoR1 | flangF3/lanspoR1 | 127F1/127R2 |
| F. sporotrichioides | Item710 | USSR | + | + | − | + |
| F. sporotrichioides | F627 | France | + | + | − | + |
| F. sporotrichioides | F95 | Poland | + | + | − | + |
| F. sporotrichioides | Item707 | Poland | + | + | − | + |
| F. sporotrichioides | Item684 | Poland | + | + | − | + |
| F. sporotrichioides | Item550 | Poland | + | + | − | + |
| F. sporotrichioides | Item194 | Italy | + | + | − | + |
| F. sporotrichioides | Item168 | France | + | + | − | + |
| F. sporotrichioides | Item196 | Italy | + | + | − | + |
| F. langsethiaea | KF2107 | Poland | + | − | + | + |
| F. langsethiaea | FSP3P/W1 | UK | + | − | + | + |
| F. langsethiaea | Item391 | Italy | + | − | + | + |
| F. langsethiaeb | CC319 | UK | + | − | + | + |
| F. langsethiaeb | CC317 | UK | + | − | + | + |
| F. langsethiaeb | CC320 | UK | + | − | + | + |
| F. langsethiaeb | CC321 | UK | + | − | + | + |
| F. langsethiaeb | CC323 | UK | + | − | + | + |
| F. langsethiaeb | CC324 | UK | + | − | + | + |
| F. langsethiaeb | CC326 | UK | + | − | + | + |
| F. langsethiaeb | CC328 | UK | + | − | + | + |
| F. langsethiaeb | CC332 | UK | + | − | + | + |
| F. langsethiaeb | 01244 | UK | + | − | + | + |
| F. langsethiaeb | C2661 | UK | + | − | + | + |
| F. langsethiaeb | 01208 | UK | + | − | + | + |
| F. tricinctum | 97FT17 | UK | − | − | − | − |
| F. sambucinum | C812 | UK | − | − | − | − |
| F. culmorum | F200 | France | − | − | − | − |
| F. graminearum | FgH | Honduras | − | − | − | − |
| F. armeniacum | I992 | USA | − | − | - | − |
| F. avenaceum | 96UKR2 | UK | − | − | − | − |
| F. chlamydosporum | F156 | Germany | − | − | − | − |
| F. crookwellense | KF334 | Germany | − | − | − | − |
| F. poae | F600 | France | − | − | − | − |
| F. flocciferum | F301 | France | − | − | − | − |
| F. proliferatum | 4854 | USA | − | − | − | − |
| F. tricinctum | CC021 | UK | − | − | − | − |
| F. equiseti | 1204 | Italy | − | − | − | − |
| F. oxysporum | Ex-5-2 | UK | − | − | − | − |
| F. crookwellense | CC001 | UK | − | − | − | − |
| M. nivale var. nivale | Mn1,1 | UK | − | − | − | − |
| M. nivale var. majus | Mn10,1 | UK | − | − | − | − |
| Species | Code | Country | spo3F/spo1R | fspo/lanspoR1 | flangF3/lanspoR1 | 127F1/127R2 |
| F. sporotrichioides | Item710 | USSR | + | + | − | + |
| F. sporotrichioides | F627 | France | + | + | − | + |
| F. sporotrichioides | F95 | Poland | + | + | − | + |
| F. sporotrichioides | Item707 | Poland | + | + | − | + |
| F. sporotrichioides | Item684 | Poland | + | + | − | + |
| F. sporotrichioides | Item550 | Poland | + | + | − | + |
| F. sporotrichioides | Item194 | Italy | + | + | − | + |
| F. sporotrichioides | Item168 | France | + | + | − | + |
| F. sporotrichioides | Item196 | Italy | + | + | − | + |
| F. langsethiaea | KF2107 | Poland | + | − | + | + |
| F. langsethiaea | FSP3P/W1 | UK | + | − | + | + |
| F. langsethiaea | Item391 | Italy | + | − | + | + |
| F. langsethiaeb | CC319 | UK | + | − | + | + |
| F. langsethiaeb | CC317 | UK | + | − | + | + |
| F. langsethiaeb | CC320 | UK | + | − | + | + |
| F. langsethiaeb | CC321 | UK | + | − | + | + |
| F. langsethiaeb | CC323 | UK | + | − | + | + |
| F. langsethiaeb | CC324 | UK | + | − | + | + |
| F. langsethiaeb | CC326 | UK | + | − | + | + |
| F. langsethiaeb | CC328 | UK | + | − | + | + |
| F. langsethiaeb | CC332 | UK | + | − | + | + |
| F. langsethiaeb | 01244 | UK | + | − | + | + |
| F. langsethiaeb | C2661 | UK | + | − | + | + |
| F. langsethiaeb | 01208 | UK | + | − | + | + |
| F. tricinctum | 97FT17 | UK | − | − | − | − |
| F. sambucinum | C812 | UK | − | − | − | − |
| F. culmorum | F200 | France | − | − | − | − |
| F. graminearum | FgH | Honduras | − | − | − | − |
| F. armeniacum | I992 | USA | − | − | - | − |
| F. avenaceum | 96UKR2 | UK | − | − | − | − |
| F. chlamydosporum | F156 | Germany | − | − | − | − |
| F. crookwellense | KF334 | Germany | − | − | − | − |
| F. poae | F600 | France | − | − | − | − |
| F. flocciferum | F301 | France | − | − | − | − |
| F. proliferatum | 4854 | USA | − | − | − | − |
| F. tricinctum | CC021 | UK | − | − | − | − |
| F. equiseti | 1204 | Italy | − | − | − | − |
| F. oxysporum | Ex-5-2 | UK | − | − | − | − |
| F. crookwellense | CC001 | UK | − | − | − | − |
| M. nivale var. nivale | Mn1,1 | UK | − | − | − | − |
| M. nivale var. majus | Mn10,1 | UK | − | − | − | − |
Received as F. sporotrichioides.
Received as F. langsethiae.
Code, origin of isolates, species name and amplification through PCR using tri5 primers (Spo3F and Spo1R), F. sporotrichioides specific primers (Fspo and LanspoR1), F. langsethiae specific primers (FlangF3 and LanspoR1) and primers to both species (127F1/127R2) of Fusarium and Microdochium species used during this study
| Species | Code | Country | spo3F/spo1R | fspo/lanspoR1 | flangF3/lanspoR1 | 127F1/127R2 |
| F. sporotrichioides | Item710 | USSR | + | + | − | + |
| F. sporotrichioides | F627 | France | + | + | − | + |
| F. sporotrichioides | F95 | Poland | + | + | − | + |
| F. sporotrichioides | Item707 | Poland | + | + | − | + |
| F. sporotrichioides | Item684 | Poland | + | + | − | + |
| F. sporotrichioides | Item550 | Poland | + | + | − | + |
| F. sporotrichioides | Item194 | Italy | + | + | − | + |
| F. sporotrichioides | Item168 | France | + | + | − | + |
| F. sporotrichioides | Item196 | Italy | + | + | − | + |
| F. langsethiaea | KF2107 | Poland | + | − | + | + |
| F. langsethiaea | FSP3P/W1 | UK | + | − | + | + |
| F. langsethiaea | Item391 | Italy | + | − | + | + |
| F. langsethiaeb | CC319 | UK | + | − | + | + |
| F. langsethiaeb | CC317 | UK | + | − | + | + |
| F. langsethiaeb | CC320 | UK | + | − | + | + |
| F. langsethiaeb | CC321 | UK | + | − | + | + |
| F. langsethiaeb | CC323 | UK | + | − | + | + |
| F. langsethiaeb | CC324 | UK | + | − | + | + |
| F. langsethiaeb | CC326 | UK | + | − | + | + |
| F. langsethiaeb | CC328 | UK | + | − | + | + |
| F. langsethiaeb | CC332 | UK | + | − | + | + |
| F. langsethiaeb | 01244 | UK | + | − | + | + |
| F. langsethiaeb | C2661 | UK | + | − | + | + |
| F. langsethiaeb | 01208 | UK | + | − | + | + |
| F. tricinctum | 97FT17 | UK | − | − | − | − |
| F. sambucinum | C812 | UK | − | − | − | − |
| F. culmorum | F200 | France | − | − | − | − |
| F. graminearum | FgH | Honduras | − | − | − | − |
| F. armeniacum | I992 | USA | − | − | - | − |
| F. avenaceum | 96UKR2 | UK | − | − | − | − |
| F. chlamydosporum | F156 | Germany | − | − | − | − |
| F. crookwellense | KF334 | Germany | − | − | − | − |
| F. poae | F600 | France | − | − | − | − |
| F. flocciferum | F301 | France | − | − | − | − |
| F. proliferatum | 4854 | USA | − | − | − | − |
| F. tricinctum | CC021 | UK | − | − | − | − |
| F. equiseti | 1204 | Italy | − | − | − | − |
| F. oxysporum | Ex-5-2 | UK | − | − | − | − |
| F. crookwellense | CC001 | UK | − | − | − | − |
| M. nivale var. nivale | Mn1,1 | UK | − | − | − | − |
| M. nivale var. majus | Mn10,1 | UK | − | − | − | − |
| Species | Code | Country | spo3F/spo1R | fspo/lanspoR1 | flangF3/lanspoR1 | 127F1/127R2 |
| F. sporotrichioides | Item710 | USSR | + | + | − | + |
| F. sporotrichioides | F627 | France | + | + | − | + |
| F. sporotrichioides | F95 | Poland | + | + | − | + |
| F. sporotrichioides | Item707 | Poland | + | + | − | + |
| F. sporotrichioides | Item684 | Poland | + | + | − | + |
| F. sporotrichioides | Item550 | Poland | + | + | − | + |
| F. sporotrichioides | Item194 | Italy | + | + | − | + |
| F. sporotrichioides | Item168 | France | + | + | − | + |
| F. sporotrichioides | Item196 | Italy | + | + | − | + |
| F. langsethiaea | KF2107 | Poland | + | − | + | + |
| F. langsethiaea | FSP3P/W1 | UK | + | − | + | + |
| F. langsethiaea | Item391 | Italy | + | − | + | + |
| F. langsethiaeb | CC319 | UK | + | − | + | + |
| F. langsethiaeb | CC317 | UK | + | − | + | + |
| F. langsethiaeb | CC320 | UK | + | − | + | + |
| F. langsethiaeb | CC321 | UK | + | − | + | + |
| F. langsethiaeb | CC323 | UK | + | − | + | + |
| F. langsethiaeb | CC324 | UK | + | − | + | + |
| F. langsethiaeb | CC326 | UK | + | − | + | + |
| F. langsethiaeb | CC328 | UK | + | − | + | + |
| F. langsethiaeb | CC332 | UK | + | − | + | + |
| F. langsethiaeb | 01244 | UK | + | − | + | + |
| F. langsethiaeb | C2661 | UK | + | − | + | + |
| F. langsethiaeb | 01208 | UK | + | − | + | + |
| F. tricinctum | 97FT17 | UK | − | − | − | − |
| F. sambucinum | C812 | UK | − | − | − | − |
| F. culmorum | F200 | France | − | − | − | − |
| F. graminearum | FgH | Honduras | − | − | − | − |
| F. armeniacum | I992 | USA | − | − | - | − |
| F. avenaceum | 96UKR2 | UK | − | − | − | − |
| F. chlamydosporum | F156 | Germany | − | − | − | − |
| F. crookwellense | KF334 | Germany | − | − | − | − |
| F. poae | F600 | France | − | − | − | − |
| F. flocciferum | F301 | France | − | − | − | − |
| F. proliferatum | 4854 | USA | − | − | − | − |
| F. tricinctum | CC021 | UK | − | − | − | − |
| F. equiseti | 1204 | Italy | − | − | − | − |
| F. oxysporum | Ex-5-2 | UK | − | − | − | − |
| F. crookwellense | CC001 | UK | − | − | − | − |
| M. nivale var. nivale | Mn1,1 | UK | − | − | − | − |
| M. nivale var. majus | Mn10,1 | UK | − | − | − | − |
Received as F. sporotrichioides.
Received as F. langsethiae.
2.2 DNA extraction and PCR amplification
For large-scale preparations from fungal isolates, 7-day-old cultures (including the PDA) were removed from their Petri-dishes, cut-up and transferred into milling-tubes. The agar–fungal material mixture was frozen and an aliquot (1 ml) of CTAB buffer (hexadecyltrimethylammonium bromide) [19] was added. The resultant mixture was ground into paste using a ball mill (Glen Creston Ltd, Stanmore; mixer/mill 8000) with steel ball bearings. DNA was extracted and purified using the CTAB buffer method modified from Nicholson et al. [20]. Milled sample was extracted in CTAB buffer (CTAB 22 mM, sarkosyl 34 mM, sorbitol 137 mM, EDTA 22 mM, polyvinylpolypyrolidone (PVPP) 1% NaCl 1.2 mM) for 60 min at 65 °C. One-third volume of 5 M Potassium acetate was added along with 1 ml chloroform:isoamyl alcohol (24:1), mixed and held at −20 °C for 20 min. The mixture was then centrifuged at 1900g for 15 min and the aqueous phase was added to two volumes of 100% ethanol. The DNA was precipitated at 850g and the pellet was washed twice with 70% (v/v) ethanol before being dissolved in TE buffer (10 mM Tris–HCl, pH 8.0, 0.1 mM EDTA).
DNA concentrations were estimated using Sybrgreen as per Hopwood et al. [10]. Aliquots of each DNA sample were added to a solution containing 100 ppm SYBR Green (Flowgen) and assayed using a Fluroskan II plate reader (INC Biomedicals Ltd, UK), which measured emission at 538 nm after excitation at 485 nm. The DNA concentration was ascertained by comparison with a serial dilution (0.0–1.8 ng μl−1) of DNA (Hind III cut λ DNA) included on each plate. DNA was diluted with TE buffer (10 mM Tris–HCl, pH 8.0, 0.1 mM EDTA) to a working concentration of ∼10 ng μl−1 and stored at −20 °C.
Genomic DNA was extracted from wheat grain samples according to the method described by Simpson et al. [21]. DNA concentrations were estimated using Sybrgreen and the samples stored at 4 °C until required.
PCR amplifications were carried out in volumes of 50 μl containing 10–20 ng of fungal DNA on a Perkin–Elmer GeneAmp PCR System 9700. PCR conditions for RAPD generation were as per Nicholson et al. [20], other species-specific PCR-assays were performed under ‘Touchdown’ cycling conditions [22]. In this process the annealing temperature was 66 °C for the first 5 cycles, and 64 °C for the next 5 cycles. For amplification of DNA from fungal cultures this was followed by 15 cycles at 62 °C while, for infected plant material, 25 cycles were carried out at this temperature. The temperature cycle used consisted of denaturation (95 °C) for 30 s, annealing (as described above) for 20 s and extension (72 °C) for 45 s with maximal ramping rates between temperatures. A final extension step of 5 min was incorporated followed by cooling to 10 °C until recovery of samples. The PCR conditions used in the amplification of ITS sequences were as per White et al. [23]. Amplification products were separated by electrophoresis through 2% agarose gels. Gels were viewed under UV light on a ‘Gel Doc 1000’ system (Bio-Rad, UK) and analysed using Molecular Analyst software (Bio-Rad, UK).
2.3 Development of primers specific to tri5
Sequence data for the design of discriminatory primers were obtained from sequence of the tri5 gene from four different Fusarium species: F. culmorum sequenced in our laboratory, F. graminearum, Genbank Accession No. AF359361, F. sporotrichioides, Genbank Accession No. AF359360, and F. sambucinum, Genbank Accession No. M64348.
After sequence comparison, regions in tri5 were targeted for designing primers with the potential for discriminating between F. sporotrichioides and other Fusarium species pathogenic to wheat. Primers were designed using the Primer Design programme in GCG, and primers selected with a theoretical melting temperature of 62 °C for amplification using ‘Touchdown’ PCR conditions [22]. These primers, designated Spo3F (5′-ATAACTCCGTGGCTGCTTGG) and Spo1R (5′-ATCAACAAACTAGATCGCTATACATT), were used to screen a number of Fusarium species.
2.4 ITS sequencing and the grouping of Fusarium isolates
Nucleotide sequences of the internal transcribed spacer (ITS) region of the ribosomal RNA gene in isolates of F. sporotrichioides and F. langsethiae, were amplified by PCR using the primers ITS4 and ITS5 [23]. PCR products were then separated by agarose gel electrophoresis, excised and sequenced. Sequence reactions were performed using the BigDye Terminator v3.0 (Applied Biosystems) system and sequences were assembled using the Staden software package for analysis with Wisconsin Package Version 10.1 (GCG) (Genetics Computer Group, Madison, WI).
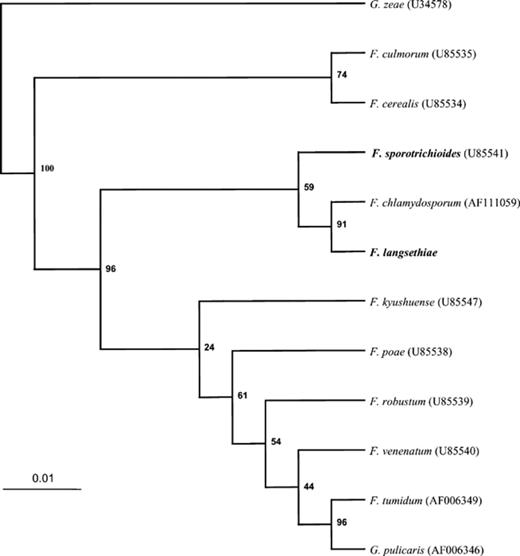
A phylogenetic tree was constructed based on these ITS sequence data using the graphical software package Phylo_Win [24]. Bootstrap data on the tree were determined with 1000 replicates using G. zeae, Genbank Accession No. U34578, as the out-group to root the tree. A total of 388 sequence sites, of which 17 were informative, were used to obtain a strict consensus tree of 46 steps generated by the maximum parsimony method, which described the phylogenetic relationship between 12 closely related Fusarium species.
2.5 Isolation and cloning of potentially discriminatory sequences
RAPD assays were performed using a number of isolates of the target species along with isolates from closely related species as determined by ITS sequence analysis. Amplification reactions were carried out in volumes of 50 μl containing 10–20 ng of fungal DNA with conditions similar to those of Nicholson et al. [14]. Decamer oligonucleotide primers from sets OP-A, OP-B, OP-T and OP-U, obtained from Operon Technologies, USA, were used to generate RAPD from those isolates placed into two distinct groups by phylogenetic analysis of the ITS. RAPD profiles were compared and bands showing either a different migratory pattern, or thought to contain sequences that could be used to discriminate between the two genetically distinct groups, were selected for cloning. These potentially discriminatory amplicons were excised and cloned into pGEM-T Easy Vectors (Promega), transformed into electro-competent E. coli (strain DH10β), according to the suppliers-instructions (Bio-Rad), and sequenced.
Sequence reactions were performed using the BigDye Terminator v3.0 (Applied Biosystems) system and sequence data were assembled using the Staden software package and analysed with Wisconsin Package Version 10.1 (GCG) (Genetics Computer Group, Madison, WI). Primers were designed using the Primer Design programme in GCG, and primers selected with a theoretical melting temperature of 62 °C for amplification using ‘Touchdown’ PCR [22] as described above.
2.6 Testing for specificity and sensitivity of species-specific primers
Amplification with primers Fspor F1 (5′-CGCACAACGCAAACTCATC) and LanspoR1 (5′-TACAAGAAGACGTGGCGATAT), designed to be specific to F. sporotrichioides, were tested for their specificity against 19 different Fusarium species. Likewise for primers FlangF3 (5′-CAAAGTTCAGGGCGAAAACT) and LanspoR1, designed to be specific to F. langsethiae, and primers 127F2 (5′-caggtagtagtagagaggtaggtaggta) and 127R2 (5′-GTCCCCAAAGAAGAAGAAGAA), designed to detect both species. All primer combinations were tested for their sensitivity against dilutions of target-species' DNA. All PCR was carried out as described above. The sensitivity of each of the species-specific primer pairs was determined by PCR amplification of DNA dilutions ranging from 30 pg to 1 fg.
3 Results
3.1 The use of tri5 sequence data to discriminate F. sporotrichioides from other Fusarium species
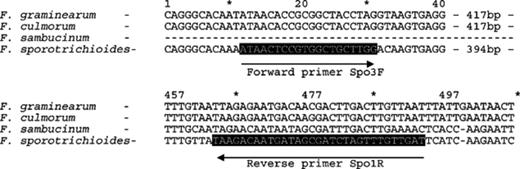
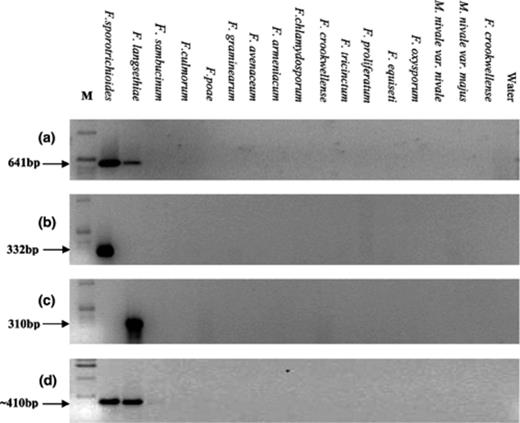
Differences between the tri5 sequences of F. sporotrichioides and those of F. graminearum, F. culmorum and F. sambucinum were exploited for their potential to be specific to F. sporotrichioides. Primers to the tri5 sequence [25] of F. sporotrichioides were designed as indicated in Fig. 1. These primers, termed Spo3F and Spo1R, were then checked for their specificity against the DNA of other Fusarium and Microdochium species and no product was produced using most non-specific DNA. However, although a high level of amplification of a product of 641 bp was achieved with DNA of F. sporotrichioides using this primer pair, DNA of F. langsethiae also resulted in a low-level of amplification of a similar sized product (Fig. 2).
A diagram showing the position of both forward (spo3F) and reverse (spo1R) primers targeted to tri5 of F. sporotrichioides.
The specificity of primer sets against 17 different Fusarium and Microdochium species (isolate name and origin are shown in Table 1). Amplification with primer sets Spo3F/Spo1R to tri5 (a); Fspo/LanspoR1, specific to F. sporotrichioides (b); FlangF3/LanspoR1, specific to F. langsethiae (c); 127F1/127F2, to both F. sporotrichioides and F. langsethiae (d).
3.2 Grouping of isolates of F. sporotrichioides and F. langsethiae
To better understand the relationship between the isolates of F. sporotrichioides and F. langsethiae used in this study, sequences of the ITS-rDNA of all isolates were determined and compared. It was found, when comparing the two groups, that invariant sequences of the ITS region differed within an 8 bp region, but were otherwise identical. A phylogram (Fig. 3), based on the ITS sequence data and that of a number of closely related Fusarium species was constructed. The ITS sequence of one group was identical to that of F. sporotrichioides (Accession No. U85541). The sequence of the second group matched an ITS sequence from a Fusarium species termed F. langsethiae (Accession No. AY220352). Subsequently, the ITS data was compared to that of other isolates of F. langsethiae and was found to be identical (Klemsdal, pers. commun.).
A phylogram displaying the relationships between ITS sequences from different species of the genus Fusarium and Gibberella. Sequences were obtained from the Genbank sequence database, Accession Numbers shown in parentheses. Sequences in bold (F. sporotrichioides and F. langsethiae), were determined by the consensus of a number of sequence reactions performed in the laboratory on different isolates. This tree was rooted in the outgroup, Gibberella zeae.
3.3 RAPDs of F. sporotrichioides and F. langsethiae
RAPD analysis was performed on nine isolates of F. sporotrichioides and four isolates of F. langsethiae using 10-mer primers to identify those producing a common amplicon in all isolates of each species [19]. Three primers produced fragment profiles showing at least one amplicon common to all isolates of F. sporotrichioides, which were not found in F. langsethiae, and vice versa (OP-B05, OP-T07 and OP-A04 respectively). These amplicons were cloned, sequenced and used to design primers.
Thirteen RAPD amplicons in total were cloned and sequenced and three were initially selected for further analysis to identify species-specific primers. Twelve primer-pairs were tested, but all of these amplified products of a similar size from both species. Primer combinations 124F1/124R2 and 124F1/124R1, however, differentially amplified products producing a high level of amplification from F. sporotrichioides and a low level from F. langsethiae. The 124F1/R1 amplicon, from both species was cloned, sequenced and compared. Sufficient sequence dissimilarity was present for the production of species-specific primers. These primers, termed FspoF1 and LanspoR1, amplified a product of 332 bp from all isolates of F. sporotrichioides tested and was shown to be specific to F. sporotrichioides (Fig. 2(b)) with no product amplification from other species. Primer pair FlangF3 and LanspoR1 amplified a product of 310 bp from all isolates of F. langsethiae tested and was shown to be specific to F. langsethiae (Fig. 2(c)) with no product amplification from other species including F. sporotrichioides. Primer pair 127F1/127F2 was designed to detect both species and amplified a product of 410 bp. The specificity of this assay was also tested against the same range of species (Fig. 2(d)). Primer pairs designed to detect F. sporotrichioides, F. langsethiae and both species were capable of detecting target sequences in total genomic DNA extractions as low as 100 fg.
The species-specific primers developed in this study were used to characterise eleven isolates from the 2001 UK wheat disease survey, co-ordinated by the Central Science Laboratory (CSL). All isolates had been identified as F. langsethiae. PCR assays confirmed that all isolates were F. langsethiae (see Fig. 4).
Amplification of products using PCR primers specific to F. langsethiae (flangF3/lanspoR1) (a) and F. sporotrichioides (fspo/lanspoR1) (b) from eleven unknown isolates resembling F. sporotrichioides[28]. All isolates were taken from the ears of winter wheat during the 2001 UK survey conducted by CSL.
4 Discussion
The tri5 gene encodes trichodiene synthase, the enzyme responsible for catalysing the production of trichodiene, the initial product in the trichothecene biochemical pathway [26]. As a common functioning unit in the production of all known Fusarium trichothecenes, the sequence of tri5 has been exploited in the development of a generic PCR-based assay to detect trichothecene-producing Fusarium species and to study trichothecene biosynthesis in Fusarium species [25]. While there is relatively little variability in the coding region of tri5 in Fusarium species [27], analysis of 5′coding- and upstream-regions of the tri5 sequence revealed a considerable amount of polymorphism. PCR primers to detect F. sporotrichioides were designed on the basis of sequence dissimilarity between this species and other Fusarium species for which sequence was available. However, these primers weakly amplified a product from F. langsethiae isolates, indicating a similarity between F. langsethiae and F. sporotrichioides in the 5′ coding- and upstream-region of tri5. This finding concurs with those of Torp and Langseth [28] in that although F. langsethiae is morphologically similar to F. poae, it has a metabolic profile resembling F. sporotrichioides and produces T-2.
Phylogenetic analyses based on 28S ribosomal DNA sequences have shown that two distinct monophyletic groups exist within the trichothecene producing Fusarium[29] and that these results correlate with classifications made on the basis of trichothecene type (A and B). To determine the phylogenetic relationship between isolates of F. sporotrichioides and those received as F. langsethiae, ITS sequence data were analysed (see Fig. 3). The most parsimonious interpretation of data confirmed that they represented two distinct, but closely related groups. These data also grouped species according to toxin production. HT-2 and T-2 type-A producers, F. sporotrichioides and F. langsethiae, grouped together and type-B trichothecene producers, F. culmorum and F. cerealis, also grouped together. Furthermore, those Fusarium species, including F. poae, that produce diacetoxyscirpenol (DAS), another type-A trichothocene, also tended to group together, but distinct from T-2 and HT-2 producing species (Fig. 3). Forward and reverse primers were designed to seven RAPD fragments, and 32 primer combinations were tested against different isolates of F. sporotrichioides and F. langsethiae. Only one primer combination was specific to F. sporotrichioides, and likewise only one was specific to F. langsethiae. This demonstrates the difficulty in developing specific primer-pairs to distinguish between these closely related Fusarium species.
F. langsethiae has been previously isolated from cereal grain in Norway [3]. Two isolates, namely KF2107 from Poland and Item391 from Italy were received as F. sporotrichioides and later found to be F. langsethiae on the basis of ITS and tri5 sequence, as well as by amplification with the FlangF3/LanspoR1 primer pair. This indicates that this species is more widespread than previously suspected and, given its ability to produce the potent T-2 and HT-2 trichothecene mycotoxins, it would need to be monitored to help establish any associated risk to human or animal health in all those areas where it has appeared. The primers designed during this study detected both species in samples of wheat grain taken from UK fields, revealing that F. langsethiae has been present in the UK since 1998. This has implications for any risk analysis, which may be undertaken for the presence of mycotoxin in UK grain.
Other results in our laboratory indicate that F. langsethiae may, in certain conditions, colonise the wheat head earlier or more rapidly than F. sporotrichioides (Simpson, unpublished). Thus, F. langsethiae may gain a competitive advantage over its close relative F. sporotrichioides through early colonisation and eventual niche exclusion. Further work is required to test this possibility, along with a study of the roles played by the environment and inter-specific competition on germination, growth, reproduction, inoculum size and dispersal, and pathogenicity of the two species.
Primers specific to both F. sporotrichioides and F. langsethiae, for use in PCR, were described here for the first time. Moreover, additional primer pairs were developed to detect both of these T-2, HT-2 producing types. The assays were used successfully in the detection of F. sporotrichioides and F. langsethiae in wheat grain taken from UK fields and to confirm the identity of isolates from UK grain.
Acknowledgements
This work was supported by the European Union through project QLK1-CT-1999-001380. The work of Paul Nicholson is supported by the Department for Enivironment, Food and Rural Affairs. Many thanks to Mona Torp and Sonia Klemsdahl for the identification and sequence comparisons of Fusarium langsethiae isolates. Thanks are also due to those people who supplied isolates for this study.
References



![Amplification of products using PCR primers specific to F. langsethiae (flangF3/lanspoR1) (a) and F. sporotrichioides (fspo/lanspoR1) (b) from eleven unknown isolates resembling F. sporotrichioides[28]. All isolates were taken from the ears of winter wheat during the 2001 UK survey conducted by CSL.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/femsle/233/1/10.1016_j.femsle.2004.01.040/1/m_FML_69_f4.jpeg?Expires=1717024850&Signature=d9ed~xELot6YqsfqGjfoqR0zGYULn84GGCZVEso687fNxmWjqSjqCjoB5NGSxEMkCJ3lwMSm8ejzOQ8UdQsUIaAG25v~ohQlVcqCAFWDUdtUROxjc1G2ztsM9RgEy4xxS9ERo5ZXI7fjqJkOXaOZU9g0~MYEKk~nl1iv5rLoiyvzVSsmSWhv83ij4QgYylebBZRic8nzN3Bie4r~Os5jKeCr9a8jJMyFfSIfSBvzuMwm4EcHMmtYhyjHjLFp3BHRu1fOf5iMzZgfFOiokBUP~AoSaXdPnoiiWMKC3gyOEHgWE91iKa6zI1JMbL3JNdBkIOC1oe0AI1zwPeTjF32Hcw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
