-
PDF
- Split View
-
Views
-
Cite
Cite
Carl-Johan Jakobsen, Flemming Søndergaard, Vibeke E. Hjortdal, Søren P. Johnsen, Use of aprotinin in cardiac surgery: effectiveness and safety in a population-based study, European Journal of Cardio-Thoracic Surgery, Volume 36, Issue 5, November 2009, Pages 863–868, https://doi.org/10.1016/j.ejcts.2009.05.040
Close - Share Icon Share
Abstract
Objective: The effectiveness and safety of aprotinin use in cardiac surgery have been questioned. More data reflecting everyday clinical practice from large-scale, unselected populations are needed. We compared the effectiveness and safety of aprotinin in cardiac surgery with those of tranexamic acid in a follow-up study using the population-based Danish health-care databases. Methods: We identified a total of 3535 patients who underwent cardiac surgery at the Aarhus University Hospital, Skejby, between 1 January 2003 and 31 December 2006; of these, 635 patients were treated with aprotinin and 2900 with tranexamic acid. We used propensity score matching to match aprotinin-treated patients with tranexamic-acid-treated patients in a 1:1 ratio, followed by Poisson regression analysis to compute relative risks (RRs). Results: Patients treated with aprotinin had more severe preoperative risk profiles than the tranexamic-acid-treated patients. The rates of postoperative drainage and transfusion of red blood cells were similar in the two groups, whereas the aprotinin group received plasma (adjusted RR = 1.39; 95% confidence interval (CI): 1.15–1.68) and platelets (adjusted RR = 1.47; 95% CI: 1.19–1.81) more frequently than the tranexamic acid group. There were no statistically significant differences in risks of re-operation due to bleeding (adjusted RR = 1.22; 95% CI: 0.84–1.78), 30-day mortality (adjusted RR = 1.03; 95% CI: 0.69–1.54), acute myocardial infarction (adjusted RR = 1.06; 95% CI: 0.69–1.64), stroke (adjusted RR = 1.36; 95% CI: 0.75–2.44) or composite major event (adjusted RR = 1.14; 95% CI: 0.87–1.50) between the two groups. However, patients who received aprotinin had an increased risk of postoperative dialysis (adjusted RR = 1.76; 95% CI: 1.15–2.70). Conclusions: Aprotinin treatment was associated with an increased use of plasma and platelet transfusion and an increased risk for postoperative dialysis, but not with other adverse outcomes, including short-term mortality.
1 Introduction
The withdrawal of aprotinin (AP) based on increased mortality amongst high-risk cardiac surgery patients in the Blood Conservation Using Antifibrinolytics in a Randomized Trial (BART) study marks a dramatic event in the saga of antifibrinolytic agents in general and AP in particular [1]. However, concerns about the safety of AP – a widely used nonspecific serine protease inhibitor – have been raised for years based on findings from a number of observational studies linking the use of AP with increased risks for renal failure, myocardial infarction (MI), stroke and death [2–5]. Concerns have been exacerbated by the apparent lack of association between other antifibrinolytic agents, including the lysine analogues tranexamic acid (TA) and ɛ-aminocaproic acid, and such adverse events [6].
Although no longer available for regular use, AP is available under a limited-use agreement for investigational use in patients with serious or immediately life-threatening diseases or conditions without other therapeutic options who are expected to benefit from the treatment. The restricted availability of AP reflects the remaining controversy and uncertainty about its effectiveness and safety in routine clinical practice. This uncertainty has been fuelled by meta-analyses—including a recent Cochrane review, based on clinical trials of antifibrinolytic therapy published before BART, the results of which are in contrast with the findings from BART and other recent observational studies [7,8]. The Cochrane review, which included data from 211 randomised clinical trials that included 20 781 participants, found reductions in blood loss and the need for allogeneic red cell transfusion and no increased risk of adverse effects, including overall mortality, in AP-treated patients as compared to those treated with lysine analogues [8].
Because other adequately sized clinical trials on AP are not likely to be conducted at this time, we must rely on previous experiences from routine clinical settings – particularly properly designed population-based studies from different settings and populations – in order to clarify the future role of aprotinin, if any. Therefore, we examined the effectiveness and safety of AP in a population-based Danish cohort study.
2 Patients and methods
We conducted this cohort study using population-based health-care databases. The Danish National Health Service provides free universal tax-supported health care to the entire population, including access to surgery.1 We used the unique personal identifier assigned to each Danish citizen at birth to link records for individuals across the databases [9]. The study was approved by The Danish Data Protection Agency.
2.1 Study population
To identify patients who underwent cardiac surgery at the Aarhus University Hospital, Skejby Sygehus, between 1 January 2003 and 31 December 2006, we used computerised data from the Western Denmark Heart Registry (WDHR). The WDHR is an Internet-based clinical registry covering all patients undergoing adult cardiac surgery in Western Denmark. Reporting to the registry has been mandatory since January 1999. Detailed patient-, surgery-, anaesthesia- and intensive care-related data are collected prospectively. Data quality is ensured by automatic validation rules at data entry combined with systematic validation procedures and random spot-checks of data after entry.
We determined that 3586 procedures were performed during the study period. For patients who underwent more than one procedure during the study period, only the most recent procedure was included (43 procedures were excluded). We excluded eight patients with an invalid personal identifier as they were, therefore, ineligible for follow-up. Ultimately, 3535 patients (98.6% of the original patient population) were included.
2.2 Aprotinin and tranexamic acid treatment
Since January 2003, all perioperative monitoring data and medications have been entered into an electronic patient management system at the Aarhus University Hospital, Skejby Sygehus. By merging data from the WDHR with data from the patient–management system, patients were classified into either the AP-treated group or the TA-treated group. General indications for AP use were re-operations, multiple cardiac procedures, aortic surgery, high co-morbidity and membership in Jehovah’s Witnesses. In all, 635 patients were treated with AP and 2900 with TA.
2.3 Anaesthesia protocols and perioperative procedures
In accordance with hospital protocol, all preoperative cardiac medications were continued until the morning of surgery, with the exception of angiotensin-converting enzyme (ACE) inhibitors and platelet inhibitors, including aspirin. However, all patients were evaluated individually and patients with acute coronary syndrome were kept on ACE inhibitors and platelet inhibitors until surgery independently of whether this was elective or acute. Patients received standard premedication in the form of benzodiazepine administered 60–90 min prior to surgery. In general, anaesthesia was administered intravenously using propofol 40–80 μg kg−1 min−1, sufentanil 3–5 μg kg−1 and pancuronium.
In the operating room, patients received routine monitoring, including five-lead electrocardiogram (ECG), radial and pulmonary artery catheters with or without continuous cardiac output measurement (Swan–Ganz CCO/VIP; Edwards Lifesciences LLC, Irvine, CA, USA), pulse oximetry, capnography and temperature monitoring. Some patients were also monitored by trans-oesophageal echocardiography.
Routine surgical and cardioprotective techniques were used for most patients, including crystalloid cardioplegia with closed cardiopulmonary bypass systems consisting of tubing with a surface-modifying additive coating, an arterial filter with heparin coating, a hollow fibre-membrane oxygenator with a surface-modifying additive coating and a venous and cardiotomy reservoir. Most patients were maintained normothermic or slightly hypothermic. At the conclusion of the surgical procedure, reperfusion of the heart was performed in accordance with the general condition of the patient and time on cross-clamp. There was no fixed postoperative treatment regimen for either pharmacological or mechanical support.
2.4 Patient and procedure characteristics
Patients and procedures were characterised by the following variables: age, age squared, sex, chronic pulmonary disease, extracardiac arteriopathy, neurological dysfunction disease, previous cardiac surgery, high preoperative serum creatinine (>200 μmol l−1), active endocarditis, critical perioperative state, unstable angina, recent MI, pulmonary hypertension, left ventricular function (three levels), emergency operation, post-infarct septal rupture together with type of surgery (five categories), diabetes, Charlson co-morbidity index (three levels) and year of surgery (two levels). We computed the EuroSCORE2 based on these variables [10]. In addition, we computed the Charlson co-morbidity index score, defining three co-morbidity levels: low (score = 0), medium (score = 1–2) and high (score ≥3) based on all discharge diagnoses recorded before the procedure [11]. We obtained the discharge diagnoses from the Danish National Hospital Registry, which records the personal identification number of the patient, dates of admission and discharge, surgical procedure(s) performed and up to 20 diagnoses classified according to the International Classification of Diseases (ICD), 8th revision until the end of 1993 and the 10th revision thereafter, for each hospital admission in Denmark since 1977 (and since 1995 for all hospital-specialist outpatient visits).
The hospital catchment area is served by pharmacies equipped with electronic accounting systems that are primarily used to secure reimbursement from the National Health Service. For each filled prescription, the patient’s civil registration number, the type and amount of drug prescribed according to the Anatomical Therapeutical Chemical classification system and the date the drug was dispensed are transferred from the pharmacies to a prescription database. We defined diabetes as a previous discharge diagnosis of diabetes and/or use of anti-diabetic medication. We used the prescription database to identify all prescriptions for insulin and oral anti-glycaemic drugs filled by the patients before the procedure [12].
2.5 Outcomes
Drug efficacy was evaluated by the volume of postoperative drainage within 48 h after surgery and the need for perioperative transfusion of blood components. Data on transfusion of blood components were obtained from the Danish Transfusion Registry [13].
The safety outcomes considered included 30-day mortality, postoperative in-hospital MI, postoperative in-hospital stroke and the need for postoperative in-hospital dialysis. Data on 30-day mortality were obtained from the Danish Civil Registration System, which keeps daily-updated records for the entire Danish population on vital status, date of death, residence and migration since 1968.
2.6 Statistical analyses
In order to overcome bias due to confounding, we matched the patients receiving AP to the patients receiving TA in a 1:1 ratio using propensity score matching [14]. We identified a set of AP- and TA-treated patients who had a similar baseline chance of being treated with AP. This was followed by model-based Poisson regression analysis with robust error variance [15] to estimate adjusted risk ratios. This two-step procedure makes the model-based inferences considerably less model dependent and generally more accurate [14]. Furthermore, the first step enables us to adjust for a large number of potential confounders since the two treatments are fairly common, [16] while the second step allows us to adjust for potential residual confounding by covariates for which the first step did not provide a satisfactory level of balance between the two treatments [17,18].
We used the R programme MatchIt3 version 2.3-1 with R4 version 2.6.1 to perform propensity score matching. The matching performed was a 1:1 nearest-neighbour match within calipers of 0.25 standard deviations (SDs) of the estimated linear propensity. We discarded observations outside the common support and then re-estimated the linear propensity score. The linear propensity scores were estimated using logistic regression including all covariates listed in Table 1 . Among these are all 17 risk factors included in the EuroSCORE [10].
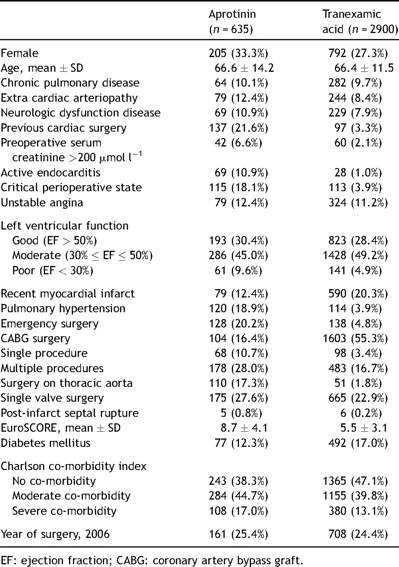
Characteristics of 3535 patients who underwent cardiac surgery at Aarhus University Hospital, Skejby between 1 January 2003 and 31 December 2006 according to treatment.
The balance of the covariates between the two treatment groups was evaluated after the initial design phase of propensity score matching to ensure that the two groups were close enough to produce reliable effect estimates. The balance was assessed using standardised differences and variance ratios [14,17]. A standardised difference with an absolute value less than 0.10 and a variance ratio between 4/5 and 5/4 was considered sufficient to support the assumption of balance of the covariate between the treatment groups [14,19]. The covariates with the least balance (i.e., variables having either an absolute standardised difference >0.05 or a variance ratio ≪5/6 or >6/5) were included in the Poisson regression, which was performed using the Statistical Analysis Software (version 9.1.3, SAS Institute Inc., Cary, NC, USA).
Data on postoperative drainage could not be assumed to follow a normal distribution. We therefore used the Wilcoxon’s rank-sum test to compare the two treatment groups.
3 Results
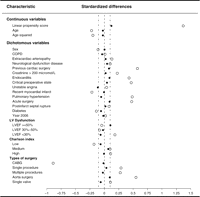
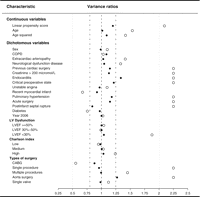
Table 1 displays patient characteristics for both the treatment groups. For all preoperative risk factors except age, recent MI, diabetes and chronic obstructive pulmonary disease, the patients treated with AP had a more adverse risk profile than the patients treated with TA. When matching on propensity score, we identified a set of 534 AP- and 534 TA-treated patients with similar patient- and procedure-related characteristics. Before matching, that is, in the full data sample, there were great imbalances between the two groups for many covariates (see Table 1). The absolute standardised differences were as high as 0.89, with 79% of the covariates having an absolute standardised difference >0.10. The most extreme variance ratio was 10.1 and 68% of the covariates had a variance ratio ≪4/5 or >5/4. After matching, the largest absolute standardised difference was 0.12 and only 11% of the covariates had an absolute standardised difference >0.10. The most extreme variance ratio was 1.34 and only 7% of the covariates had a variance ratio ≪4/5 or >5/4. Thus, the propensity score matching largely removed the imbalances in the covariates (Figs. 1 and 2 ).
Standardised differences in variables included in the propensity score for the entire study population (light dots) and for the propensity score-matched patients (black dots).
Variance ratios of variables included in the propensity score for the entire study population (light dots) and for the propensity score-matched patients (black dots).
There were no statistically significant differences between the treatment groups in the volume of postoperative drainage during the first 48 h in the intensive care unit. In the overall study population, the median volumes were 563 and 600 ml in the AP and TA groups, respectively (p = 0.16). A similar pattern was found in the smaller propensity score-matched study population (p = 0.14). Within the AP group, we found a significantly higher proportion of patients with low-volume postoperative drainage (i.e., less than 400 ml) (34.2% vs 25.4%, chi-square test, p = 0.0047). However, the AP group also had a larger proportion of patients with a high-volume drainage (i.e., >1000 ml) (28.3% vs 22.7%) (chi-square test, p = 0.0024).
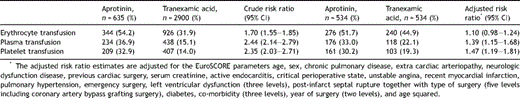
A higher overall proportion of AP-treated patients received transfusion with erythrocytes, plasma and platelets as compared to TA-treated patients (Table 2 ). However, the transfusion requirements among AP-treated patients remained higher only for plasma and platelets when restricting the analysis to propensity score-matched patients and adjusting for possible confounding factors. The overall risk of re-operation due to bleeding was higher among patients receiving AP than among patients receiving TA. Among the propensity-matched patients, 10.1% of the AP-treated patients required re-operation due to bleeding, whereas 8.2% of the TA-patients required re-operation. This difference corresponded to an adjusted relative risk (RR) of 1.22 (95% confidence interval (CI): 0.84–1.78).
Transfusion of blood components in the per- and postoperative period. Crude risk ratio is based on the entire study population and the adjusted risk ratio is based on propensity score-matched patients.
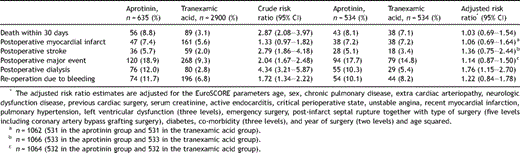
Patients who received AP had substantially higher crude risks of all adverse outcomes than did patients who received TA (Table 3 ). However, when restricting the analyses to propensity score-matched patients and adjusting for possible confounding factors, we found no substantial differences in the risk of 30-day mortality (adjusted RR = 1.03; 95% CI: 0.69–1.54), in-hospital MI (adjusted RR = 1.06; 95% CI: 0.69–1.64), in-hospital stroke (RR = 1.36; 95% CI: 0.75–2.44) or composite major event (RR = 1.14; 95% CI: 0.87–1.50) between the AP- and TA-treated patients. In contrast, patients treated with AP had a greater risk of requiring postoperative dialysis (adjusted RR = 1.76; 95% CI: 1.15–2.70) than the patients treated with TA.
Risk ratio estimates for postoperative outcomes among patients treated with aprotinin as compared to patients treated with tranexamic acid. The crude risk ratio estimates are based on all patients, whereas the adjusted risk ratio estimates are based on propensity score-matched patients. Postoperative major event includes death, myocardial infarct, and stroke.
4 Discussion
In this population-based cohort, we found that adult cardiac surgery patients treated with AP had greater plasma- and platelet-transfusion requirements and an increased risk for postoperative dialysis as compared to patients treated with TA. We found no substantially increased risk of other adverse outcomes, including 30-day mortality, in-hospital MI, in-hospital stroke or a composite outcome of death, MI or stroke.
The main strength of our study is its prospective population-based design with complete follow-up for a broad range of clinical outcomes and consequently low risk of selection and information bias. Detailed and complete data on patient- and procedure-related characteristics were available and thorough efforts were made, including the combination of propensity score matching and multivariable adjustment techniques, to minimise any impact of confounding on the findings. Despite these strengths, the results should be interpreted with caution. The study was observational and, although we considered a wide range of covariates during design and analysis, we cannot exclude the possibility that residual confounding due to the use of crude variables (e.g., presence of co-morbidities) or unknown/unmeasured prognostic factors, including lifestyle factors, may have confounded the results. Furthermore, even though our underlying study population was relatively large, the moderate use of AP resulted in a well-balanced but moderately sized sample of propensity score-matched patients.
Our findings with regard to blood loss and requirements for transfusion indicate that the effectiveness of AP in preventing or reducing bleeding in routine clinical practice is not greater than that of TA. These findings are at least partly in accordance with findings from a Cochrane review [8], other observational studies [2,20] and the BART study [1], which reported a moderate to no effect of AP on blood loss and massive bleeding as compared with other systemic antifibrinolytic agents. The increased use of plasma and thrombocytes may partly be explained by the fact that more patients in the TA group had postoperative bleeding >1000 ml, which is the more likely to be substituted than patients bleeding ≪400 ml postoperative. However, we cannot entirely exclude that patients receiving AP may be considered more prone to bleeding and thus the threshold for additional haemostatic products may have been lower. The most recent Cochrane review (which did not include data from the BART study) not only found an advantage of AP over the lysine analogues TA and ɛ-aminocaproic acid in terms of operative blood loss, but also noted that the differences were small [8]. AP also appeared superior in reducing the need for erythrocyte transfusion (RR = 0.83; 95% CI: 0.69–0.99) and re-operation due to bleeding when compared with the lysine analogues. However, the authors found indications of publication bias; adjustment for these effects reduced the magnitude of estimated benefits such that the apparent advantage of AP over the lysine analogues was small or explainable by publication bias and non-equivalent drug doses [8]. The risk of publication bias was underlined by the fact that the Cochrane review identified no less than 211 randomised clinical trials, of which some had very small sample sizes [8]. Other observational studies have reported more marked differences in efficacy in favour of AP; [21,22] however, it appears that the scientific evidence supporting the low efficacy of AP in reducing blood loss is increasing. This poor efficacy is particularly troublesome in light of the high cost of AP as compared to the lysine analogues [23].
Questions with regard to the safety of AP include concerns about cardiovascular and cerebrovascular complications, renal failure and short- and long-term mortality [1–5,20]. The concerns were initially raised in observational studies but were recently confirmed in the BART study, which found a higher 30-day mortality rate for AP-treated patients as compared to TA- and ɛ-aminocaproic acid-treated groups (RR = 1.53; 95% CI: 1.06–2.22). The majority of deaths were from cardiac causes, including cardiogenic shock, MI and right ventricular failure [1]. We found no statistically significant differences in 30-day mortality, postoperative MI, stroke, or a combined end-point of these events, but an almost two-fold increased risk for postoperative dialysis. These findings mirror those previously reported by Karkouti and colleagues, who, in a study designed similarly to ours (i.e., single-centre study, propensity score-matched ‘real-life’ patients treated with AP or TA), found AP to be associated with an increased risk of renal dysfunction defined as a >50% increase in creatinine concentration during the first postoperative week (>100 μmol l−1 in women, >110 μmol l−1 in men or a new requirement for dialysis support) [20]. In the BART study, a borderline statistically significant increase in the risk of doubling the baseline creatinine level (RR = 1.50; 95% CI: 0.97–1.40) was found among AP-treated patients; however, no consistent effect of AP on renal failure was detected [1]. The Cochrane review reported a RR of renal dysfunction from the remaining available trials of 1.16 (95% CI: 0.79–1.70) when comparing AP-treated patients with patients treated with lysine analogues. However, cautious interpretation was recommended because renal events appeared to have been underreported in the clinical trials of AP [8]. Thus, the data with regard to the effect of AP on renal function remain contradictory [24]. However, while some observational studies have not reported AP to be associated with adverse effects [21,22,25], a clear majority of observational studies using propensity score-matching methods to eliminate confounding, including ours, link AP with various serious adverse effects [2–5,20].
In conclusion, we found no evidence of reduced bleeding compared to patients receiving TA and an increased use of plasma and platelet transfusion among adult cardiac surgery patients treated with AP. In addition, AP use was associated with an increased risk for postoperative dialysis, but not with other adverse outcomes, including short-term mortality.
Part of data presented in poster form at the 23rd International Conference on Pharmacoepidemiology & Therapeutic Risk Management, Quebec City, Canada, 19–22 August 2007.
References
EuroSCORE: http://euroscore.org.
Matching as Nonparametric Preprocessing for Parametric Causal Inference, 2004. http://gking.harvard.edu/matchit/.
A Language and Environment for Statistical Computing. 2007. http://www.R-project.org.
- myocardial infarction, acute
- aprotinin
- hemodialysis
- perioperative cardiovascular risk
- cardiac surgery procedures
- hemorrhage
- blood platelets
- cerebrovascular accident
- ischemic stroke
- erythrocyte transfusion
- follow-up
- hospitals, university
- plasma
- platelet transfusion
- repeat surgery
- safety
- tranexamic acid
- dialysis procedure
- mortality
- health care