-
PDF
- Split View
-
Views
-
Cite
Cite
Erich Stoelben, Corinna Ludwig, Chest wall resection for lung cancer: indications and techniques, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 3, March 2009, Pages 450–456, https://doi.org/10.1016/j.ejcts.2008.11.032
Close - Share Icon Share
Summary
Lung cancer invasion of the chest wall is not a common challenge and represents only about 5% of all patients resected for lung cancer. In T3N0M0 tumours, long-term survival reaches 40–50%, provided certain conditions are fulfilled. The number of explorative thoracotomies and the rate of non-radical resections might be high due to the local extension of an aggressive tumour. Mortality after resection is as high as for pneumonectomy. For historical and anatomical reasons, we have to divide the patients into two groups: infiltration of, and above, the second rib (Pancoast) and tumours located caudally to the second rib. We have to define the two entities. There is a problem concerning correct diagnosis: many tumours reach the chest wall. If the lung is not adherent to the parietal pleura, a standard lobectomy can be performed. However, in the case of adhesions, the differentiation between tumour invasion and inflammation may be difficult. We do not want to perform over-treatment since lung resection en bloc with the chest wall has a higher morbidity and mortality than lobectomy. But we have to avoid opening the tumour intraoperatively or perform a non-radical resection. Therefore, we need a preoperative diagnostic tool answering the question of extrapulmonary infiltration. In this context, we will discuss whether extrapleural lung resection is acceptable in the case of pleural invasion without chest wall involvement. The prognosis of patients with tumours invading the chest wall and mediastinal lymph node metastasis is worse. But patients with ipsilateral supraclavicular lymph node metastasis are not excluded. Thus, careful clinical investigations are necessary. To achieve complete resection, the surgeon should use anatomical knowledge to choose the best form of access to make radical resection more feasible. The problem of pain after thoracotomy is accentuated after chest wall resection, especially after paravertebral resection. The use of modern pain treatment is very important.
1 Clinical presentation and diagnostics
1.1 History, definition and epidemiology
The surgical treatment of lung cancer invading the chest wall started with the report by Coleman in 1947 [1]. Up until now the published single-centre series has not exceeded 200 patients [2–10]Table 1 . The proportion of lung cancers invading the chest wall from all resected lung cancers is about 5%. Only 1% are so called Pancoast or sulcus superior cancers.
Literature overview of results in patients with lung cancer and chest wall resection.
The Pancoast tumour [11,12] was described in 1924 as a tumour of unknown origin, infiltrating the apex of the chest with destruction of bone and clinical signs of nerve injury like pain, Horner’s syndrome and muscle atrophy of the hand. Curative resection was first described by Chardack and MacCullum in 1956 [13] by en bloc resection and adjuvant radiotherapy. In 1956, Shaw and Paulson started the treatment of patients and published results in 1961 [14]. They combined preoperative radiotherapy followed by resection, learning from the poor experience of adjuvant radiotherapy after non-radical resection. At the time of publication, five out of nine patients were still alive disease-free for more than 1 year. The Pancoast tumour is a subgroup of lung cancers invading the chest wall and covers about 20% of all chest wall invading tumours.
In this early publication, pain was the clinical sign leading patients to consult their doctors. The pain was burning and continuous (Paulson called it ‘excruciating’) and did not respond effectively to high doses of analgesics. Today, peripheral lung cancers invading the parietal pleura which do not cause chest pain are detected by other clinical signs (e.g. pneumonia or haemoptysis) or incidentally, by extensive use of chest radiographs. In the studies cited below, pain is mentioned by about 50% of the patients with chest wall invasion.
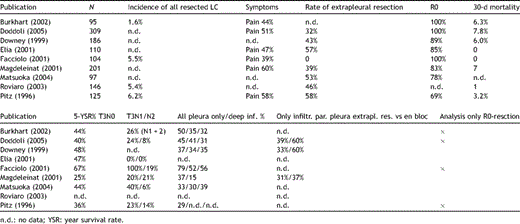
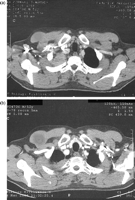
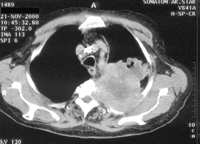
Lung cancers invading the parietal pleura in the apex of the thorax have to be treated like classical Pancoast tumours with neurological symptoms. That is the reason why the definition of the entity Pancoast tumour had to be renewed, since pain is no longer the primary clinical sign of a Pancoast tumour. A new definition was recommended by Detterbeck in 2003 [15]: lung cancer invading the parietal pleura or chest wall at the level of the second rib or above is called a Pancoast or sulcus superior tumour. Following this definition, in patients without Pancoast’s syndrome (Horner’s syndrome, pain, bone destruction and atrophy of hand muscles), the diagnosis of Pancoast tumour depends only on the infiltration of the parietal pleura. However, this early stage of infiltration may be difficult to clarify even intraoperatively. Therefore, in the study published by Rusch et al. [16], preoperative radio-chemotherapy was administered only in patients with Pancoast’s syndrome or infiltration of bone or infraclavicular vessels demonstrated by MRI or a CT scan (Figs. 1–4 ).
Pancoast tumour T3N0M0 right (a) before and (b) after radio-chemotherapy, access Shaw–Paulson, lobectomy, resection of the 1st to 3rd rib in combination with the transversal process and the 1st thoracic nerve root.
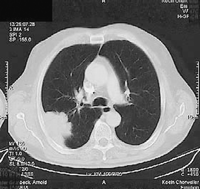
Squamous cell lung cancer with infiltration of the soft tissue of the chest wall (pT3pN0M0), posterolateral access, lobectomy, resection of the 4–8th rib.
Squamous cell cancer (pT2pN0M0) without fixation to the parietal pleura, anterolateral access, lobectomy.
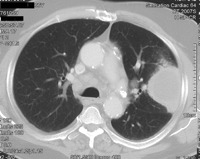
Squamous cell cancer (T4N0M0) infiltrating the chest wall and the spine.
1.2 Diagnostic procedures
The preoperative evaluation comprises the standardised examinations for functional resectability using spirometry in cases of normal or near normal lung function. In patients with compromised lung function diffusion capacity, perfusion scan and spiro-ergometry are indicated. There are no lung function limits for extended lung and chest wall resection, although the risk of mortality after extended lobectomy is about three times higher (6%) than after standard lobectomy. Therefore, the clinical assessment by the surgeon is an important contribution to the decision of functional resectability.
Histology has to be obtained by transbronchial biopsy or transthoracic needle aspiration before non-surgical treatment as well as extended lung resection.
The oncological resectability is defined by staging for mediastinal lymph node and distant metastases. Since the prognosis of patients with mediastinal lymph node metastases and chest wall infiltration is worse (T3N2M0, < 10%), the histological proof of N2 disease should lead to the recommendation of definitive non-surgical treatment such as radio-chemotherapy. In the case of Pancoast tumours, ipsilateral supraclavicular lymph node metastases do not preclude trimodality treatment.
PET has proven to be an effective tool to stage lung cancer concerning lymph nodes and distant sites. It can be recommended as a basic investigation technique in combination with MRI of the brain. The suspicion of mediastinal lymph node metastases has to be confirmed by cytological or histological examination [15].
Since every lung cancer in contact with the chest wall carries the possibility of chest wall infiltration, the surgeon should carefully check this possibility to be able to plan the optimal treatment for the patients.
Careful history taking and clinical examination with special attention for signs of local tumour extension and systemic tumour effects (weight loss, etc.) is crucial for decision-making regarding further diagnostic steps or therapeutic decisions. In patients with tumours infiltrating the chest wall caudally to the second rib, pain is documented in about 50% of cases. Pain is a highly specific (>90%) clinical sign for chest wall infiltration.
In patients with superior sulcus tumours, irradiating pain (chest wall, shoulder and arm), Horner’s syndrome (miosis, ptosis, enopthalmus and anhydrosis), functional defects of the brachial plexus (from ulnar nerve palsy to high level cross-section) and vascular complications (venous occlusion) can occur [12]. A preoperative neurological investigation to define pre-existing defects is recommended.
1.3 Preoperative investigations to define local invasion
Four clinical situations are possible when the tumour reaches the chest wall:
The tumour infiltrates the soft tissue or bones of the chest wall. Complete chest wall resection is clearly necessary to achieve a radical operation.
The tumour has only penetrated the visceral and parietal pleura. Extrapleural lobectomy might be possible for histologically documented complete resection.
The tumour and the visceral pleural are fixed to the parietal pleura by inflammatory adhesion induced by the tumour or by previous pleurisy. Extrapleural lobectomy is sufficient for radical resection.
The lung and the tumour are not fixed to the chest wall. Standard lobectomy would be an adequate operation.
The intraoperative decision in cases two and three can be difficult, but radicality, postoperative morbidity and long-term survival are dependent on a correct decision.
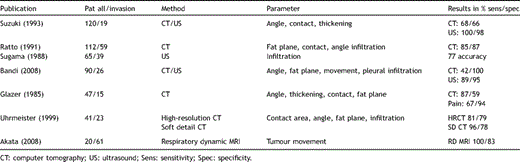
CT scan, MRI, ultrasound and bone scan may answer the question whether the parietal pleura and chest wall are infiltrated [17–23]. Sensitivity and specificity range from 60 to 100% depending on the method, the parameters used and the selection of patients. The difference between T2 (infiltration of visceral pleura) and T3 with infiltration of the parietal pleura or the soft tissue of the chest wall may be as small as 1 mm. Furthermore, the area of infiltration may be small and missed by radiological or even pathological investigations (Table 2 ).
Some data suggest that full-thickness resection of the chest wall, in the case of infiltration of the parietal pleura only, results in a higher 5-year survival rate than after extrapleural lobectomy. In most of the series published up until now, the decision of performing extrapleural lobectomy or chest wall resection was made intraoperatively by the surgeon trusting in his personal experience. Indeed, the rate of extrapleural resection ranges from 30 to 60%. We recommend being aware of a T3 tumour in every case of a tumour making contact with the parietal pleura and if in doubt to use further investigation such as high-resolution CT, MRI or ultrasound. Secondly, if the surgeon finds the tumour fixed to parietal pleura and this fixation is not obviously produced by an inflammatory reaction, parietal infiltration is likely. In these cases, full-thickness resection of the chest wall is recommended to reach a higher 5-year survival rate.
In Pancoast tumours, MRI is recommended for anatomical visualisation of local invasion to plan the extent of resection and reconstruction. Infiltration of the vertebral body, brachial plexus and infraclavicular vessels is possible [24].
1.4 TNM
Using the TNM definition for local invasion, in Pancoast tumours, differentiation between T3 and T4 is not easy to handle. In the publications cited below, the definition of T3 and T4 tumours is not discussed in detail.
Infiltration of the parietal pleura or chest wall (with or without destruction of bone), the intercostal nerves and mediastinal pleura is defined as T3.
Infiltration of the vertebral body and great vessels is defined as T4. Therefore, the infiltration of the mediastinal vessels in the upper part of the mediastium (subclavian artery, innominate vein, vena cava) leads to stage IIIB.
2 Treatment
2.1 Pre- and postoperative oncological treatment
Pancoast tumours were the first thoracic oncological entities treated by combined modality. Radical resection was exceedingly difficult to achieve and adjuvant radiotherapy after non-radical resection did not prove to be successful. Shaw and Paulson changed the timing of this bimodality treatment (radiotherapy followed by surgery), obtaining a higher number of radical resections with long-term clinical success. The introduction of combined radio-chemotherapy in locally advanced lung cancer gave rise to studies using this tri-modal setting in Pancoast tumours. This phase II studies in Pancoast tumours demonstrated the best results after radio-chemotherapy in these tumours until now [16,25–27].
Randomised studies in locally advanced lung cancers (stages IIIA–IIIB) comparing induction treatment and surgery (tri-modality) with radio-chemotherapy alone did not clearly favour surgery, probably due to a high pneumonectomy and non-radical resection rate [28,29]. The overall oncological results in trimodality treatment of Pancoast tumours are better probably because patients with N2 disease are excluded and, for this challenging subgroup of patients, a high rate of radical resection is performed. Thus trimodality treatment is the standard of care for Pancoast tumours. Adjuvant treatment after radio-chemotherapy and surgery is not indicated.
Since the number of patients is small, we have no clinical data published until now about the pretreatment of lung cancers invading the chest wall below the second rib. In cases with large tumours or N1 disease, a neoadjuvant treatment to reduce tumour size and lymph node infiltration would be helpful to achieve radical resection. The results after resection of T3N2 tumours are not satisfying, with 5-year survival rate not exceeding 10%. Therefore, if N2 disease is proven histologically, surgery is not recommended. T3N0 and T3N1 tumours belong to stage II, a group of patients for which adjuvant chemotherapy is intended with 5-year survival benefit of about 5% absolute [30].
The value of postoperative radiotherapy is unclear. It is used after radical or non-radical resection, but its influence on the oncological results is not documented. Since the clinical benefit of adjuvant radiotherapy after radical resection of lung cancer is not proven [31], we do not recommend it after complete resection. After non-radical resection or after extra pleural lobectomy of tumours infiltrating the parietal pleura only (with doubtful radicality) postoperative radiotherapy can be considered, but there is no evidence to prove this.
2.2 Planning the thoracotomy
The surgical access to the thorax could be crucial for the objective of radical resection of chest wall infiltrating lung cancer. Most of the cancers arise from the dorsoapical part of the upper or lower lobe of the lungs. Therefore, tumours tend to infiltrate the paravertebral portion of the ribs. In our experience, these posteriorally located tumours are resected more comfortably using a posterior access. Accordingly, we use the anterolateral approach (our standard for lung resection) for lung cancers that invade the chest wall anteriorly to the mid axillary line. For chest wall resection beyond this line, we use the posterolateral approach. This allows excellent exposure of the spine and makes resection of the transverse process and the vertebral bodies easier.
The same rule can be applied to Pancoast tumours. If they infiltrate the dorsal part of the upper sulcus (plexus, ganglion stellatum and vertebral body), the extended access, used by Shaw and Paulson, allows paravertebral rib resection, spinal surgery and lung resection with standard lymphadenectomy. In tumours infiltrating the first two ribs in the anterior part of the superior sulcus, the surgeon can expect involvement of the subclavian vessels. In these cases, the anterior approaches described by Dartevelle (anterior transcervical thoracic approach) [32] or Masaoka, using partial sternotomy [33], are helpful. Using the transcervical approach, resection of the medial part of the clavicle gives access to the dissection of the subclavian vessels and their confluence with their cervical partners (innominate vein and brachiocephalic artery). In cases where involvement of the vena cava or the mediastinal part of the subclavian artery (left) or the brachiocephalic artery (right) is suspected, sternotomy allows better vascular control by clamping the vessels near their origin.
2.3 Lobectomy or sublobar resection in Pancoast tumours?
In the recently published prospective studies, surgeons mainly used lobectomy for lung resection after radio-chemotherapy. This is not supported by the retrospective studies, which described wedge resections and lobectomies and found no unanimously statistical benefit for lobectomy [34,35]. Nevertheless, lobectomy is indicated in lung cancer as a standard for resection. The good results after segmental resection in small peripheral lung cancers cannot be transferred to the patients discussed above.
2.4 Extended resections (vessels and spine)
One third of the patients in the recent studies are classified as having T4 tumours. In contrast, the number of extended resections (spine and vessels) is about 10%. One supposes that the tumour regression after radio-chemotherapy could help to avoid complete vertebral body or vessel replacement. Irrespective of this experience, the thoracic surgeon in cooperation with a neurosurgeon is able to solve difficult situations using anterior and posterior access to the spine. This may help to delay or even prevent local complications like paraplegia [36,37].
2.5 Chest wall reconstruction
The first three ribs are well covered by the pectoral muscle and the shoulder plate and do not need any replacement. After resection of the lower ribs, we use a simple monofilament net made of polypropylene, which is placed over the osseous chest wall defect and supports the chest wall muscle. This net reduces the respiratory movements. The net drains the wound secretions to the intrathoracic drainage. In the case of infection the monofilament structure of the net is incorporated into infected tissue, a clinical problem that may occur in patients with a prolonged air leak. An alternative for chest wall reconstructions is PTFE. We do not recommend the use of rigid prosthesis, because they tend, induced by the continuing respiratory movements, to break and penetrate the surrounding tissue.
2.6 Pain management
The tumour infiltrating the chest wall and the intercostal nerve often induces neuropathic pain. This kind of pain can also be aroused after thoracotomy in up to 50% of patients [38]. The clinical experience suggests an even higher prevalence of neuropathic pain after multisegmental chest wall resection. Therefore, the treatment of pain is a very important part of chest wall surgery. As the chronic pain is attributable to an injury to the intercostal nerve, gentle preparation of the nerve, no unnecessary resection and, if inevitable, minimal traumatic cut of the nerve should be considered. Depending on the guidelines and internal agreements, the use of local methods like peridural anaesthesia or intercostal nerve block, application of opioids in sufficient amounts or by patient-controlled analgesia and the early employment of specific analgesic for the treatment of neuropathic pain are recommended. Effective pain treatment prevents early morbidity such as mucus retention and pneumonia and has a crucial impact on the quality of life after chest wall resection.
2.7 Morbidity and mortality and late results
The median of postoperative mortality is about 6%. This risk is three times higher than after standard lobectomy. The main cause of mortality was respiratory insufficiency in 42 of 62 patients, who died after lung and chest wall resection in the studies cited below (Table 1). This can be due to a higher rate of acute respiratory insufficiency syndrome after extended resection or due to retention of bronchial secretions, impaired coughing and pneumonia. Therefore, postoperative care of these patients has to be attentive.
In 11–31% of the patients, a radical resection of the lung cancer was not possible. The survival of these patients is poor.
In the follow-up, isolated local relapse appears in 10–16% of patients, whereas distant metastases are the leading cause of death. Forty-five to 50% of patients are still living 5 years after radical resection in the case of N0 disease. The finding of lymph node metastases deteriorates survival to 20% in N1 and to under 10% in N2 disease. These results confirm the role of effective neo- and adjuvant chemotherapy.
In most of the studies, the grade of invasion of the tumour into the parietal pleura or the chest wall has no influence on survival. However, in these results, the influence of the extension of resection (extrapleural lobectomy vs full-thickness resection) and invasion are mixed. The combination of depth of invasion and extension of surgery is produced by a mechanism of chance (or intraoperative decision by the surgeon). In three studies, tumours with infiltration of the parietal pleura only are analysed and in two of them a beneficial effect of full-thickness resection of the chest wall is proven. Since the difference between invasion and breakthrough of the parietal pleura is very small, extrapleural lobectomy in the case of infiltration of the parietal pleura is a doubtful radical operation.
Radical resection of Pancoast tumours after radio-chemotherapy was possible in about 75% of the patients. The locoregional relapse rate is low at 9–15%, comparable to the results after chest wall resection.
3 For the future
It is necessary to ameliorate preoperative diagnosis of tumour stage and local invasion.
Reduce the rate of exploratory thoracotomy and to increase the rate of radical resections.
Investigate neoadjuvant and adjuvant radiotherapy for locally advanced cancers.
Decrease the overall postoperative mortality rate.
Use the benefit of adjuvant chemotherapy.
Acknowledgements
This work is dedicated to Professor Jochen Hasse, an outstanding instructor in thoracic surgery, who taught and guided us to perfection and compassion.
References
- inflammation
- lung
- adhesions
- neoplasm metastasis
- pain
- pancoast's syndrome
- pneumonectomy
- preoperative care
- ribs
- thoracoplasty
- thoracotomy
- pain management
- diagnosis
- mediastinum
- morbidity
- mortality
- neoplasms
- pleura
- lung volume reduction
- lung cancer
- lobectomy
- lymph node metastasis
- chest wall
- clinical diagnostic instrument
- supraclavicular lymph node
- tumor cell invasion
- overtreatment