Abstract
We investigated the effects of daily ultraviolet A1 (UV-A1, 340–400 nm) exposures on mood states (#R19055, approval on 21 October 2020). Based on our earlier findings of the influence of diurnal preference on mood, we investigated further whether diurnal preference plays a role in the influence of UV-A1 on mood states. Forty-one healthy participants aged 19–55 years were randomized to receive either UV-A1 (n = 21) or control (n = 20) exposures (violet light, 390–440 nm). The irradiations were administered on three consecutive mornings on the skin of the buttocks and middle back. Diurnal preference was assessed with the modified 6-item Morningness-Eveningness Questionnaire (mMEQ). Changes in mood were assessed with Total Mood Disturbance (TMD) score of the 40-item Profile of Mood States (POMS) before the first irradiation, immediately after each irradiation and one week after the last irradiation. Mood improved among those subjected to UV-A1 exposures compared with the controls (p = 0.031). Individuals with more pronounced morningness had mood improvement (p = 0.011), whereas those with more pronounced eveningness did not (p = 0.41). At follow-up of one week after the last irradiation the mood improvement had disappeared.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Ultraviolet radiation (UV-R), its harms and benefits to humans as well as its influence on skin physiology have been investigated for long. The solar UV-R reaching the earth’s surface consists of 5–10% of ultraviolet B (UV-B, 280–315 nm) and 90–95% of ultraviolet A (UV-A, 315–400 nm), thus UV-A being the main component of terrestrial UV-R [1]. The effects on molecules, cells and tissues of UV-B differ from those of UV-A. More energetic UV-B requires lower doses for skin sunburn and causes long-lasting and lightly protective tanning through the accumulation of melanin in epidermal keratinocytes, while UV-A is a source of immediate short-term erythema and pigmentation [1, 2], especially in darker skin types [3]. However, both UV-B and UV-A are considered carcinogens and cause DNA damage principally directly and indirectly, respectively. UV-B is mainly absorbed by the epidermis, the outermost layer of the skin [1]. UVA penetrates deeper into the underlying dermis and subcutis [4]. The immunomodulating properties of longwave UV-A (UV-A1, 340–400 nm) are used for treatment of certain immune-mediated dermatological conditions, such as sclerosing and cutaneous connective tissue diseases. The UV-A2 (315–340 nm) spectrum is filtered out from the UV-A1 light sources to reduce adverse effects resembling those of UV-B, such as erythema [5,6,7].
The mood enhancing effect of exposure to UV-R has been proposed to involve the production of β-endorphin via stimulation of the pro-opiomelanocortin promoter [8,9,10,11], in addition in vivo trial [12]. However, all of the studies provide no support for the hypothesis that UV-R exposures increase the circulating β-endorphin in humans [13,14,15,16]. Alternatively, cosmetic tanning is an explanation for the positive feelings. Tanners tend to feel relaxed and less tense, and this phenomenon may explain why tanned skin is desirable [17, 18]. Even though UV-B is the cause of delayed and more persistent skin tanning, UV-A1 is the primary UV-R component of indoor tanning beds [2].
Diurnal preference (morningness to eveningness) measures the individual’s preferred timing for daily activities, and individuals are of the morning, intermediate, or evening type by their diurnal preference [19]. The individual diurnal preference remains rather constant throughout adulthood, but with aging it tends to drift towards the morning hours [20]. There is a peripheral circadian clock in fibroblasts, keratinocytes, and melanocytes of the human skin [21], and diurnal preference is associated with the interpersonal variation in output measures related to the circadian clock, e.g., in dermal fibroblast cell cultures [22]. Earlier, we discovered that in healthy volunteers exposed to whole-body narrow-band UV-B (NB-UV-B, 309–313 nm) on four consecutive afternoons the diurnal preference towards eveningness was associated with mood improvement [23]. Currently, the effects of UV-A1 on mood, if any, are not known.
Our current study herein investigated whether UV-A1 (340–400 nm) exposures induce changes in mood states compared with control exposures to violet light (390–440 nm), and whether the diurnal preference contributes to changes in mood states.
2 Materials and methods
The Regional Medical Ethics Committee of Wellbeing Services County of Pirkanmaa, Finland, approved the study protocols (#R19055, approval on 21 October 2020). The study was conducted adhering to the Declaration of Helsinki and its amendments. No compensation was given for participating in the study. All the participants gave their written informed consent.
The estimated number of participants needed for this experimental study was based on our earlier studies with NB-UV-B [24], as well as on earlier research using questionnaires for profile of mood states [25]. The exclusion criteria included a diagnosis of depression or depressive symptoms within three months prior to the study; antidepressant, immunosuppressant, neurological or photosensitizing medication within three months prior to the study; photosensitivity; Fitzpatrick’s anamnestic skin type I, V, or VI [26]; pregnancy or lactation; regular smoking; a history of skin cancer or premalignant lesions; extensive scarring or keloid tendency and less than one month since last significant exposure to ultraviolet radiation (solarium or sunny holiday). In addition, the participants were advised to avoid sauna and ice swimming two days before as well as during the study.
Diurnal preference was assessed with the modified version (mMEQ; [27]) of the original Morningness-Eveningness Questionnaire (MEQ; [19]). Shortened versions of the MEQ produce satisfactory to excellent correlations with the original MEQ which includes 19 items [28]. Our modified version contained six items (4, 7, 9, 15, 17, and 19) of the original MEQ, which explains about 83% of the variance in the original MEQ [27]. High scores indicate the preference towards morningness and low scores towards eveningness. Based on the total score of 5–27, we assigned the participants to having their preference more towards morningness (score of > 14, hereafter called morningness) or more towards eveningness (score of ≤ 14, hereafter called eveningness).
2.1 Procedure
The trial was implemented in the Department of Dermatology and Allergology, Tampere University Hospital (Tampere, Finland) from December 2020 to beginning of the April 2021, and from the end of October 2021 to December 2021, when ambient UV-A1 exposure is low as well as the skin is protected by clothing.
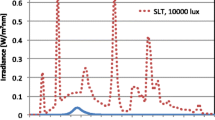
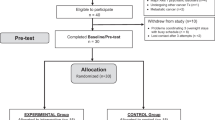
Participants were randomized into either exposure to the (UV-A1, 340–400 nm) part-body irradiations, or exposure to the control (violet light, 390–440 nm) part-body irradiations. Each participant received a total of three part-body irradiations, one irradiation in three consecutive mornings each between 7:00 h to 10:00 h. The irradiations were administered with the medical Sellamed 4000 UV-A 1-Part-body Radiation System (System Dr. Sellmeier, 4.2 kW-Unit, Sellas Medizinische Geräte GmbH, Ennepetal, Germany) from the distance of 30 cm of the skin surface on the buttocks and middle back (at the level of the kidneys). The combination of filters and reflectors allows emission of almost solely UV-A1 radiation in the range of 340–400 nm (97%) and that of 340–440 nm (> 99%), whereas radiation of shorter wavelengths than 320 nm is eliminated totally. The spectral irradiance was measured using Ocean Optics S2000 spectroradiometer (Ocean Optics, Dunedin, FL; see Fig. 1). The dose rate for the exposures was measured using Airam UVM-8 UV-meter (calibrated specifically for Sellamed 4000 spectrum, calibration factor 1.08), and the power density was 79.9 mW/cm2. The needed irradiation time required for the 10 J/cm2 UV-A1 dose was determined according to this measurement (10 J/cm2 divided by 79.9 mW/cm2 = 125 s = 2 min 5 s). Control irradiations were administered with the same device and procedure, but using a removable, transparent polycarbonate glass to filter the shorter-wave ultraviolet radiation, and thus the control group received visible (violet) light (390–440 nm). The cumulative doses were 30 J/cm2 for UV-A1 (340–400 nm) and 0.42 J/cm2 (0.14 J/cm2 in three consecutive mornings) for control (390–440 nm) irradiation (measurement in person by the author LY for this study). The device was preheated for two minutes before every irradiation. During preheating, the participants rested in position and their skin were covered with a removable, impermeable blanket. The participants rested in position during and for three minutes after the irradiation, when the device was cooling down. To prevent participants from seeing to which group they were randomized and to avoid any eye-related adverse events, the eyes were closed from the beginning of the preheating to the end of the resting time. The device was covered by a blanket before and immediately after the irradiations.
2.2 Assessment of mood states
Change in mood states was assessed with the Profile of Mood States (POMS) administered before the irradiations (baseline), immediately after each daily irradiation (primary outcome), and one week after the last irradiation (follow-up). We used the modified version of the POMS, which contains 40 items and seven distinct mood subscales [29], instead of the original version containing 65 items [30]. The POMS elicits responses to one-word items on a 5-point scale from “0 = Not at all, 1 = A little, 2 = Moderately, 3 = Quite a lot, to 4 = Extremely”, to indicate “How are you feeling at the moment?” The two esteem-related affect items (embarrassed, ashamed) are reverse-scored prior to being combined with the other items. A Total Mood Disturbance (TMD) score is calculated by summing the total scores for the five negative subscales (tension, depression, fatigue, confusion, anger) and then subtracting the total scores for the two positive subscales (vigor, esteem-related affect). A constant of 100 was added to the TMD formula to avoid negative scores.
2.3 Statistical analysis
The primary outcome was the change in the TMD scores immediately after the three daily irradiations compared to the baseline. The results are reported as means with their standard deviations (SD) and 95% confidence intervals (CIs). The Fisher’s exact test (categorial data) or the Mann–Whitney U-test (continuous data) was performed for intergroup comparisons. Repeated measures were analyzed using a bootstrap (Monte-Carlo with 10,000 replications) approach for generalized estimating equations (GEE; Gaussian distribution and an identity link function) using an exchangeable correlation structure. The fixed effects included group, time (days: 0, 1, 2 and 3), and group × time interactions. Effect sizes (d) were calculated using the method of Cohen (D), where an effect size of 0.20 is considered small, 0.50 moderate, and 0.80 large, and the 95% CIs for the effect sizes were obtained by bias-corrected bootstrapping (5000 replications). No adjustment for multiple comparisons was considered necessary, as there was only one primary analysis and a small sample size. All analyses were performed using STATA 17.0 (StataCorp, College Station, TX).
3 Results
A total of 41 volunteers participated in the study. To describe the sample, there was no difference in gender (Fisher’s exact test: p = 0.35), age (Mann–Whitney U test: p = 0.90), mMEQ score (Mann–Whitney U test: p = 0.91) nor Fitzpatrick’s anamnestic phototype (Pearson chi-square test: p = 0.58) between the participants assigned to the two intervention groups, but by the body-mass index (BMI) they differed (Mann–Whitney test: p = 0.031).
In the UV-A1 group, there were 21 individuals, 14 women and seven men, aged 19–52 y (mean = 30, SD = 8, median = 28), having BMI of 23.0 on average (SD = 3.3) and mMEQ score of 16.6 on average (SD = 3.9), seven with Fitzpatrick’s anamnestic phototype II and 14 with phototype III. 14 assigned to the morningness and seven to the eveningness (Table 1).
In the control group (visible violet light), there were 20 individuals, 10 women and 10 men, aged 20–55 years (mean = 32, SD = 11, median = 29), having BMI of 26.1 on average (SD = 4.5) and mMEQ score of 16.8 on average (SD = 8), six with anamnestic skin phototype II, 14 with phototype III and one with phototype IV. 11 assigned to the morningness and nine to the eveningness (Table 1).
There was a significant difference in the TMD score change from the baseline between the groups (GEE model with Wald test p = 0.031; Fig. 2). The decrease in the change in the TMD scores from baseline was greater in the UV-A1 group than in the control group after the irradiations, thus implying a greater positive change in mood after receiving UV-A1 exposures (Table 2).
TMD change from baseline. TMD score changes on average with the 95% CI from baseline as a function of day 1, day 2 and day 3 after exposure to UV-A1 (340–400 nm) and control, visible violet light (390–440 nm) irradiations. The left panel shows all the participants (UV-A1, n = 21; control, n = 20), the middle panel the morningness individuals (UV-A1, n = 14; control, n = 11), and the right panel the eveningness individuals (UV-A1, n = 7; control, n = 9). The effect sizes were 0.61 (95% CI: 0.05 to 1.36) for all participants, 0.91 (95% CI: 0.10 to 2.10) for those assigned as having morningness, and 0.33 (95% CI: − 0.67 to 1.35) for those assigned as having eveningness. Repeated measures were analyzed by using generalized estimating equations (GEE) with Wald test. TMD total mood disturbance
Further, there was a significant difference in the TMD score change from baseline among those having their diurnal preference toward morningness (GEE model with Wald test p = 0.011; Fig. 2). The positive impact on mood based on the TMD score change from baseline was greater after the UV-A1 exposures than after the control irradiations among those having their diurnal preference toward morningness. However, there was no significant change among those having their diurnal preference toward eveningness (GEE model with Wald test p = 0.41; Fig. 2). The effect sizes were 0.61 (95% CI: 0.05 to 1.36) for all participants, 0.91 (95% CI: 0.10 to 2.10) for those assigned as having morningness, and 0.33 (95% CI: -0.67 to 1.35) for those assigned as having eveningness.
From baseline TMD scores to TMD scores one week after the last exposure (follow-up TMD scores), there was no difference (GEE model with Wald test p = 0.75) in the TMD score change between the control and UV-A1 groups (Table 3).
4 Discussion
We found that mood was enhanced by UV-A1 exposures as compared to control exposures, and that the effect was greater among the individuals with their diurnal preference toward morningness rather than toward eveningness. Earlier studies have reported many differences between morning-type and evening-type of persons [see, e.g., 31], and the diurnal preference towards eveningness is associated with a small, but significant effect size with depression [32]. We have reported earlier that the more the diurnal preference was inclined toward eveningness, the greater the improvement in mood was over the five days after the four daily sub-erythematous NB-UV-B exposures on afternoons [23], and that individuals having their diurnal preference toward morningness were more prone to erythema induced by NB-UV-B exposure in the evening than those toward eveningness [33].
For a systematic review on the effects of UV-R on mood, Veleva et al. [34] found three trials, where exposure was administered on the skin, which all reported that UV-R had positive effects on mood, but all these trials bore bias due to their study design as well as publication bias was not excluded. Two of these trials administered UV-A, but none assessed the diurnal preference. First, Gambichler et al. [14] studied 53 healthy volunteers of whom 42 participants had six whole-body UV-A exposures within the following three weeks, whereas 11 participants had none. The six exposures to UV-A, with the cumulative dose of UV-A of 96 J/cm2 for Fitzpatrick’s II skin types and 126 J/cm2 for Fitzpatrick’s III skin types, resulted in the feelings of being significantly more strengthened, more balanced, and less nervous. Second, Edström et al. [35] studied 22 healthy volunteers of whom 4 participants had whole-body UV-B and 6 participants had whole-body UV-A exposures with the median of 17 UV-B or UV-A exposures, whereas 12 participants had whole-body placebo (visible white light, with 0.24 mW/cm2 UV-A and 0.018 mW/cm2 UV-B, for 10 min) exposures with the median of 10 placebo exposures. These groups were irradiated twice to thrice a week, and there was no significant effect on mood.
In our current study, we found a positive change in mood immediately after the three daily exposures to UV-A1 in the morning, thus the dose cumulating to 30 J/cm2. Our dose was thus substantially smaller than that in the study of Gambichler et al. [14] and the effect we achieved was transient, as the positive impact faded within a week. The impact on mood might have been greater if higher doses of UV-A1 or an extended series of exposures had been used. On the hand, prolonged UV-R irradiation increases the hazards of UV-A1, such as photoaging and photocarcinogenesis of the skin [36, 37]. On the other hand, a randomized controlled study design would not have worked any longer, as the appearance of erythema and pigmentation after frequent and high-dose exposures would have revealed the nature of our light source. Further, in our study, had the irradiations been administered in the afternoon or evening, their effect on mood might have been different, but more research is needed to verify or nullify this question.
The exposures to violet light or UV-A1 of 10 J/cm2 did not cause erythema or tanning visible to the naked eye within 15 to 20 min after the irradiations nor 24 h after the first and the second irradiations. We chose to administer the UV-A1 dose of 10 J/cm2 each morning, because no visible erythema nor pigmentation was desired. The UV-A1 dose of 10 J/cm2 we used for each exposure equals the 45-min exposure to sunlight in summer in the city of Tampere, Finland, at the latitude of 61°N (calculation by the author LY for the study). Since exposure to a dose of 10 J/cm2 of UV-A1 would take about 80 min in the autumn or the spring at the latitude of 61°N in Tampere and over 9 h in the winter (calculation by the author LY for the study), the only source of UV-A1 for the participants during the study was the device.
UV-A1 is not highly erythematic, and to define the barely erythema, that is the minimal erythemal dose (MED) of the skin, it may have required even a dose of 61 J/cm2 from the Sellamed 4000 device (calculation by the author LY for the study). The devices commonly used for the definition of the MED include rays of both UV-B and UV-A2. These shorter wavelengths than UV-A1 are the main causative factors for erythema of the skin, and the rays of UV-A1 alone are therefore not used for the MED test. The minimal pigmentary dose of UV-A is much lower than the MED, and exposures to the UV-A dose of greater than 10 J/cm2 may cause immediate pigment darkening which fades in two hours as well as persistent pigment darkening which may last for 24 h [38]. The pigmentation of the skin after UV-A1 and visible light exposures has been shown for Fitzpatrick’s skin types IV–VI [3] and higher (50 J/cm2) single doses of UVA1 for Fitzpatrick’s skin types III–IV [39].
Our findings herein suggest that the mood-enhancing effect of UV-A1 may be mediated via a cutaneous pathway, e.g., the spinoparabrachial pathway which exposure to heat as well as UV-B (302 nm) can activate, leading to mood enhancement due to activation of subsets of serotonergic neurons in the brain but, on the other hand, to sunburn and pain with overexposure to UV-B [40,41,42,43]. Shorter UV-B wavelengths transduce via transient receptor potential cation channels (subfamily V, member 4) in keratinocytes, whereas longer UV-A wavelengths transduce via transient receptor potential cation channels (subfamily A, member 1) in melanocytes [40] but not in keratinocytes [44]. It may be that exposures to UV-A1, with a daily non-erythematic dose over three days avoiding the overexposure, activate the spinoparabrachial pathway via skin-innervating sensory neurons, whereas the daily exposures to violet light of less than 1 J/cm2 are not energetic enough to activate the pathway.
As optical radiation is absorbed into the skin, the absorption results in some heat in the skin [45, 46], so the warming effect might explain the positive change in mood after exposures to UV-A1 as well. However, this explanation is unlikely, as absorption of visible violet light which we used for control exposures also results in warmth in the skin. Finally, as the vitamin D synthesis in the skin requires wavelengths shorter than 330 nm [47] which we did not use for the exposures, the mood-enhancing effect of UV-A1 cannot be explained by induction of vitamin D synthesis. Instead, the opsins (OPN1SW, OPN2, OPN3 and OPN5) in the keratinocytes and melanocytes, absorbing the wavelengths of less than 400 nm, provide a potential nexus for photons dissipated as heat energy and rays of UV-A1 to interact, which may induce physiological responses [48, 49]. Of them, rhodopsin (OPN2) is activated not only in melanocytes by UV-A (320–400 nm) more than UV-B (280–320 nm) [50], but also in keratinocytes by violet light (380–420 nm) [51], and thus might be a target of high interest in future studies (see Fig. 3).
Schematic of possible mechanisms of action. The mood-enhancing effect of UV-A1 may be mediated via a cutaneous pathway. Mechanistically, we hypothesize that A the circadian clock components in the skin cells react to UV-A1 wavelengths and activate the spinoparabrachial pathway via skin-innervating sensory neurons. B The opsins in the skin provide a possible nexus for photons dissipated as heat energy and rays of UV-A1 to interact and induce the mood-enhancing effect of UV-A1
Our study has limitations as well. A greater number of participants would have enabled to analyze whether the chronotype (morning, intermediate, evening) contributes to changes in mood states among the UV-A1 group compared to control exposures. Here, due to the limited group sizes, we divided the diurnal preference towards morningness or eveningness by the mMEQ midpoint score. Follow-up studies with greater sample size are needed. As a function of the time of day, there is a routine fluctuation in psychological processes [52], concerning the subjective evaluations of mood as well, even with questionnaires of rather permanent aspects over time. We did not measure this. Furthermore, the subjective feeling of distinctness of the diurnal fluctuations has its amplitude that relates to health status, suggesting that the greater this amplitude is, the worse the health status is [53]. We did not measure this either. Here, it is of note that in our study the participants were healthy volunteers without depressive symptoms. Therefore, more studies are needed to investigate mood improvement among those who suffer from depression or other psychiatric conditions. Skin biopsies and blood samples are needed to elucidate the relationships of cutaneous, humoral and neural factors as mechanisms of action in mood regulation.
5 Conclusion
To conclude, our study suggests that skin exposure to UV-A1 can enhance mood states. However, long-term mood enhancement likely requires greater or daily repeated exposures.
Data availability statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request only.
7 References
D’Orazio, J., Jarrett, S., Amaro-Ortiz, A., & Scott, T. (2013). UV radiation and the skin. International Journal of Molecular Sciences, 7(14), 12222–12248. https://doi.org/10.3390/ijms140612222
Tewari, A., Grage, M. M., Harrison, G. I., Sarkany, R., & Young, A. R. (2013). UVA1 is skin deep: Molecular and clinical implications. Photochemical & Photobiological Sciences, 12, 95–103. https://doi.org/10.1039/c2pp25323b
Mahmoud, B. H., Ruvolo, E., Hexsel, C. L., Liu, Y., Owen, M. R., Kollias, N., Lim, H. W., & Hamzavi, I. H. (2010). Impact of long-wavelength UVA and visible light on melanocompetent skin. The Journal of Investigative Dermatology, 130, 2092–2097. https://doi.org/10.1038/jid.2010.95
Finlayson, L., Barnard, I. R. M., McMillan, L., Ibbotson, S. H., Brown, C. T. A., Eadie, E., & Wood, K. (2022). Depth Penetration of light into skin as a function of wavelength from 200 to 1000 nm. Photochemistry and Photobiology, 98, 974–981. https://doi.org/10.1111/php.13550
York, N. R., & Jacobe, H. T. (2010). UVA1 phototherapy: A review of mechanism and therapeutic application. International Journal of Dermatology, 49, 623–630. https://doi.org/10.1111/j.1365-4632.2009.04427.x
Gambichler, T., & Schmitz, L. (2018). Ultraviolet al Phototherapy for Fibrosing Conditions. Front Med (Lausanne), 5, 237. https://doi.org/10.3389/fmed.2018.00237
Prasad, S., Coias, J., Chen, H. W., & Jacobe, H. (2020). Utilizing UVA-1 Phototherapy. Dermatologic Clinics, 38, 79–90. https://doi.org/10.1016/j.det.2019.08.011
Levins, P. C., Carr, D. B., Fisher, J. E., Momtaz, K., & Parrish, J. A. (1983). Plasma beta-endorphin and beta-lipoprotein response to ultraviolet radiation. Lancet, 2, 166. https://doi.org/10.1016/s0140-6736(83)90150-2
Wintzen, M., Yaar, M., Burbach, J. P., & Gilchrest, B. A. (1996). Proopiomelanocortin gene product regulation in keratinocytes. The Journal of Investigative Dermatology, 106, 673–678. https://doi.org/10.1111/1523-1747.ep12345496
Skobowiat, C., Dowdy, J. C., Sayre, R. M., Tuckey, R. C., & Slominski, A. (2011). Cutaneous hypothalamic-pituitary-adrenal axis homolog: Regulation by ultraviolet radiation. American journal of physiology. Endocrinology and metabolism, 301, E484–E493. https://doi.org/10.1152/ajpendo.00217.2011
Slominski, A. T., Zmijewski, M. A., Skobowiat, C., Zbytek, B., Slominski, R. M., & Steketee, J. D. (2012). Sensing the environment: Regulation of local and global homeostasis by the skin’s neuroendocrine system. Advances in Anatomy, Embryology and Cell Biology, 212, 1–115. https://doi.org/10.1007/978-3-642-19683-6_1
Jussila, A., Huotari-Orava, R., Ylianttila, L., Partonen, T., & Snellman, E. (2016). Narrow-band ultraviolet B radiation induces the expression of β-endorphin in human skin in vivo. Journal of Photochemistry and Photobiology B: Biology, 155, 104–108. https://doi.org/10.1016/j.jphotobiol.2016.01.007
Wintzen, M., Ostijn, D. M., Polderman, M. C., le Cessie, S., Burbach, J. P., & Vermeer, B. J. (2001). Total body exposure to ultraviolet radiation does not influence plasma levels of immunoreactive beta-endorphin in man. Photodermatology, Photoimmunology and Photomedicine, 17, 256–260. https://doi.org/10.1034/j.1600-0781.2001.170602.x
Gambichler, T., Bader, A., Vojvodic, M., Avermaete, A., Schenk, M., Altmeyer, P., & Hoffmann, K. (2002). Plasma levels of opioid peptides after sunbed exposures. British Journal of Dermatology, 147, 1207–1211. https://doi.org/10.1046/j.1365-2133.2002.04859.x
Gambichler, T., Bader, A., Vojvodic, M., Bechara, F. G., Sauermann, K., Altmeyer, P., & Hoffmann, K. (2002). Impact of UVA exposure on psychological parameters and circulating serotonin and melatonin. BMC Dermatology, 12, 2–6. https://doi.org/10.1186/1471-5945-2-6
Kaur, M., Liguori, A., Fleischer, A. B., Jr., & Feldman, S. R. (2006). Plasma beta-endorphin levels in frequent and infrequent tanners before and after ultraviolet and non-ultraviolet stimuli. Journal of the American Academy of Dermatology, 54, 919–920. https://doi.org/10.1016/j.jaad.2006.01.062
Feldman, S. R., Liguori, A., Kucenic, M., Rapp, S. R., Fleischer, A. B., Jr., Lang, W., & Kaur, M. (2004). Ultraviolet exposure is a reinforcing stimulus in frequent indoor tanners. Journal of the American Academy of Dermatology, 51, 45–51. https://doi.org/10.1016/j.jaad.2004.01.053
Sivamani, R. K., Crane, L. A., & Dellavalle, R. P. (2009). The benefits and risks of ultraviolet tanning and its alternatives: The role of prudent sun exposure. Dermatologic Clinics, 27(149–54), vi. https://doi.org/10.1016/j.det.2008.11.008
Horne, J. A., & Östberg, O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. International Journal of Chronobiology, 4, 97–110.
Broms, U., Pitkäniemi, J., Bäckmand, H., Heikkilä, K., Koskenvuo, M., Peltonen, M., Sarna, S., Vartiainen, E., Kaprio, J., & Partonen, T. (2014). Long-term consistency of diurnal-type preferences among men. Chronobiology International, 31, 182–188. https://doi.org/10.3109/07420528.2013.836534
Sandu, C., Dumas, M., Malan, A., Sambakhe, D., Marteau, C., Nizard, C., Schnebert, S., Perrier, E., Challet, E., Pévet, P., & Felder-Schmittbuhl, M. P. (2012). Human skin keratinocytes, melanocytes, and fibroblasts contain distinct circadian clock machineries. Cellular and Molecular Life Sciences, 69, 3329–3339. https://doi.org/10.1007/s00018-012-1026-1
Ferrante, A., Gellerman, D., Ay, A., Woods, K. P., Filipowicz, A. M., Jain, K., Bearden, N., & Ingram, K. K. (2015). Diurnal preference predicts phase differences in expression of human peripheral circadian clock genes. J Circadian Rhythms, 13, 4. https://doi.org/10.5334/jcr.ae
Toledo, A., Karppinen, T., Miettinen, M. E., Leppäluoto, J., Vuolteenaho, O., Ylianttila, L., Kautiainen, H., Snellman, E., & Partonen, T. (2019). Narrow-band ultraviolet B (NB UV-B) exposures improve mood in healthy individuals differently depending on chronotype. Chronobiology International, 36, 1570–1580. https://doi.org/10.1080/07420528.2019.1661424
Nikkola, V., Grönroos, M., Huotari-Orava, R., Kautiainen, H., Ylianttila, L., Karppinen, T., Partonen, T., & Snellman, E. (2018). Circadian time effects on NB-UVB-induced erythema in human skin in vivo. The Journal of Investigative Dermatology, 138, 464–467. https://doi.org/10.1016/j.jid.2017.08.016
Annesi, J. J. (2005). Changes in depressed mood associated with 10 weeks of moderate cardiovascular exercise in formerly sedentary adults. Psychol Rep. https://doi.org/10.2466/pr0.96.3.855-862
Fitzpatrick, T. B. (1988). The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology, 124, 869–871. https://doi.org/10.1001/archderm.124.6.869
Hätönen, T., Forsblom, S., Kieseppä, T., Lönnqvist, J., & Partonen, T. (2008). Circadian phenotype in patients with the co-morbid alcohol use and bipolar disorders. Alcohol and Alcoholism, 43, 564–568. https://doi.org/10.1093/alcalc/agn057
Kanagarajan, K., Gou, K., Antinora, C., Buyukkurt, A., Crescenzi, O., Beaulieu, S., Storch, K.-F., & Mantere, O. (2018). Morningness-Eveningness questionnaire in bipolar disorder. Psychiatry Research, 262, 102–107. https://doi.org/10.1016/j.psychres.2018.02.004
Grove, J. R., & Prapavessis, H. (1992). Preliminary evidence for the reliability and validity of an abbreviated Profile of Mood States. International Journal of Sport Psychology, 23, 93–109.
McNair DM, Lorr M, Droppleman LF. (1971). Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service.
Fabbian, F., Zucchi, B., De Giorgi, A., Tiseo, R., Boari, B., Salmi, R., Cappadona, R., Gianesini, G., Bassi, E., Signani, F., Raparelli, V., Basili, S., & Manfredini, R. (2016). Chronotype, gender and general health. Chronobiology International, 33, 863–882. https://doi.org/10.1080/07420528.2016.1176927
Norbury, R. (2021). Diurnal preference and depressive symptomatology: A meta-analysis. Science and Reports, 11, 12003. https://doi.org/10.1038/s41598-021-91205-3
Raita, A., Häggqvist, I.-M., Joronen, H., Nikkola, V., Huotari-Orava, R., Ylianttila, L., Kautiainen, H., Snellman, E., Pasternack, R., & Partonen, T. (2022). Diurnal preference contributes to maximal UVB sensitivity by the hour of the day in human skin in vivo. The Journal of Investigative Dermatology, 142, 2289–91.e5. https://doi.org/10.1016/j.jid.2022.01.021
Veleva, B. I., van Bezooijen, R. L., Chel, V. G. M., Numans, M. E., & Caljouw, M. A. A. (2018). Effect of ultraviolet light on mood, depressive disorders and well-being. Photodermatology, Photoimmunology and Photomedicine, 34, 288–297. https://doi.org/10.1111/phpp.12396
Edström, D. W., Linder, J., Wennersten, G., Brismar, K., & Ros, A. M. (2010). Phototherapy with ultraviolet radiation: A study of hormone parameters and psychological effects. Journal of the European Academy of Dermatology and Venereology, 24, 403–409. https://doi.org/10.1111/j.1468-3083.2009.03423.x
Battie, C., Jitsukawa, S., Bernerd, F., Del Bino, S., Marionnet, C., & Verschoore, M. (2014). New insights in photoaging, UVA induced damage and skin types. Experimental Dermatology, 1, 7–12. https://doi.org/10.1111/exd.12388
Bernerd, F., Passeron, T., Castiel, I., & Marionnet, C. (2022). The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. International Journal of Molecular Sciences, 23, 8243. https://doi.org/10.3390/ijms23158243
Sklar, L. R., Almutawa, F., Lim, H. W., & Hamzavi, I. (2013). Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochemical & Photobiological Sciences, 12, 54–64. https://doi.org/10.1039/c2pp25152c
Marionnet, C., Nouveau, S., Hourblin, V., Pillai, K., Manco, M., Bastien, P., Tran, C., Tricaud, C., de Lacharrière, O., & Bernerd, F. (2017). UVA1-induced skin darkening is associated with molecular changes even in highly pigmented skin individuals. The Journal of Investigative Dermatology, 137, 1184–1187. https://doi.org/10.1016/j.jid.2016.12.016
Moore, C., Cevikbas, F., Pasolli, H. A., Chen, Y., Kong, W., Kempkes, C., Parekh, P., Lee, S. H., Kontchou, N. A., Yeh, I., Jokerst, N. M., Fuchs, E., Steinhoff, M., & Liedtke, W. B. (2013). UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci USA, 110, E3225–E3234. https://doi.org/10.1073/pnas.1312933110
White, J. P. M., Cibelli, M., Urban, L., Nilius, B., McGeown, J. G., & Nagy, I. (2016). TRPV4: Molecular conductor of a diverse orchestra. Physiological Reviews, 96, 911–973. https://doi.org/10.1152/physrev.00016.2015
Hale, M. W., Dady, K. F., Evans, A. K., & Lowry, C. A. (2011). Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Experimental Neurology, 227, 264–278. https://doi.org/10.1016/j.expneurol.2010.11.012
Hale, M. W., Raison, C. L., & Lowry, C. A. (2013). Integrative physiology of depression and antidepressant drug action: Implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacology & Therapeutics, 137, 108–118. https://doi.org/10.1016/j.pharmthera.2012.09.005
Atoyan, R., Shander, D., & Botchkareva, N. V. (2009). Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. The Journal of Investigative Dermatology, 129, 2312–2315. https://doi.org/10.1038/jid.2009.58
Diffey BL, Kochevar IE. (2007). Basic principles of photobiology. In: Lim HW, Hönigsmann H, Hawk JL, (Eds.), Photodermatology. (pp. 15–27), New York: Informa healthcare.
Mahmoud, B. H., Hexsel, C. L., Hamzavi, I. H., & Lim, H. W. (2008). Effects of visible light on the skin. Photochemistry and Photobiology, 84, 450–462. https://doi.org/10.1111/j.1751-1097.2007.00286.x
Commission Internationale de l’Éclairage (CIE). (2006). Action spectrum for the production of previtamin D3 in human skin. CIE, 174, 2006.
Haltaufderhyde, K., Ozdeslik, R. N., Wicks, N. L., Najera, J. A., & Oancea, E. (2015). Opsin expression in human epidermal skin. Photochemistry and Photobiology, 91, 117–123. https://doi.org/10.1111/php.12354
de Assis, L. V. M., Tonolli, P. N., Moraes, M. N., Baptista, M. S., & de Lauro Castrucci, A. M. (2021). How does the skin sense sun light? An integrative view of light sensing molecules. Journal of Photochemistry and Photobiology, C: Photochemistry Reviews, 47, 100403. https://doi.org/10.1016/j.jphotochemrev.2021.100403
Wicks, N. L., Chan, J. W., Najera, J. A., Ciriello, J. M., & Oancea, E. (2011). UVA phototransduction drives early melanin synthesis in human melanocytes. Current Biology, 21, 1906–1911. https://doi.org/10.1016/j.cub.2011.09.047
Kim, H.-J., Son, E. D., Jung, J.-Y., Choi, H., Lee, T. R., & Shin, D. W. (2013). Violet light down-regulates the expression of specific differentiation markers through rhodopsin in normal human epidermal keratinocytes. PLoS ONE, 8, e73678. https://doi.org/10.1371/journal.pone.0073678
Adan, A. (1993). Circadian variations in psychological measures: A new classification. Chronobiologia, 20, 145–161.
Díaz-Morales, J. F., Randler, C., Arrona-Palacios, A., & Adan, A. (2017). Validation of the MESSi among adult workers and young students: General health and personality correlates. Chronobiology International, 34, 1288–1299. https://doi.org/10.1080/07420528.2017.1361437
Acknowledgements
We thank all the participants of this study and translator Virginia Mattila for providing language help. Further, we want to thank Finnish Medical Foundation and the Finnish Dermatological Society for personal grants to the author AH.
Funding
Open access funding provided by Tampere University (including Tampere University Hospital). This work was supported by the Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospital [#9X052] and AH personal grants from the Finnish Medical Foundation and the Finnish Dermatological Society.
Author information
Authors and Affiliations
Contributions
Conceptualization: AH, RP, HK, LY, ES, TP; data curation: AH, RP, HK, ES, TP; formal analysis: HK; funding acquisition: AH, RP, ES; investigation: AH, RP, LY, ES, TP; methodology: RP, LY, ES, TP; project administration: AH, RP, LY, ES, TP; resources: AH, RP, HK, LY, ES, TP; supervision: RP, LY, ES, TP; validation: AH, RP, HK, LY, ES, TP; visualization: AH, HK, LY; writing- original draft preparation: AH, ES, TP; writing—review and editing: AH, RP, HK, LY, TP, ES.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haapasalo, A., Pasternack, R., Kautiainen, H. et al. Influence of ultraviolet A1 exposures on mood states: a randomized controlled study. Photochem Photobiol Sci (2024). https://doi.org/10.1007/s43630-024-00587-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43630-024-00587-6