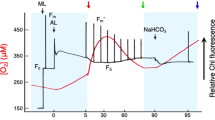
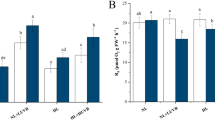
Abstract
The photosynthetic apparatus is a major reactive oxygen species (ROS) proliferator, especially in high-light environments. The role of ROS in photoinhibition and photoacclimation mechanisms has been extensively explored, primarily in model plant species. However, little work has been performed on the topic in non-Archaeplastida organisms, such as the model heterokont species Nannochloropsis oceanica. To investigate the photoacclimation and damaging impact of singlet oxygen and superoxide anions on the photosynthetic apparatus of N. oceanica, we subjected cells to two doses of methyl viologen and rose bengal. Significant findings: Rose bengal (a singlet-oxygen photosensitizer) induced changes to the photosynthetic apparatus and PSII photochemistry mirroring high-light-acclimated cells, suggesting that singlet-oxygen signaling plays a role in the high-light acclimation of PSII. We further suggest that this singlet-oxygen pathway is mediated outside the plastid, given that rose bengal caused no detectable damage to the photosynthetic apparatus. Methyl viologen (a superoxide-anion sensitizer) induced an enhanced non-photochemical quenching response, similar to what occurs in high-light-acclimated cells. We propose that superoxide anions produced inside the plastid help regulate the high-light acclimation of photoprotective pathways.
Graphical abstract






Similar content being viewed by others
References
Salvatori, N., Alberti, G., Mulle, O., & Peressotti, A. (2022). Does fluctuating light affect crop yield? A focus on the dynamic photosynthesis of two soybean varieties. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2022.862275
Perin, G., Gambaro, F., & Morosinotto, T. (2022). Knowledge of regulation of photosynthesis in outdoor microalgae cultures is essential for the optimization of biomass productivity. Frontiers in Plant Science, 13, 1–11.
Foyer, C. (2018). Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environmental and Experimental Botany, 154, 134–142.
Bashir, F., Rehman, A. U., Szabó, M., & Vass, I. (2021). Singlet oxygen damages the function of Photosystem II in isolated thylakoids and in the green alga chlorella sorokiniana. Photosynthesis Research, 149, 93–105.
Nishiyama, Y., Allakhverdiev, S. I., Yamamoto, H., Hayashi, H., & Murata, N. (2004). Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry, 43, 11321–11330.
Bhatt, M., Pandey, S. S., Tiwari, A. K., & Tiwari, B. S. (2021). Plastid-mediated singlet oxygen in regulated cell death. Plant Biology, 23, 686–694.
Gururani, M. A., Venkatesh, J., & Tran, L. S. P. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Molecular Plant, 8, 1304–1320.
Nishiyama, Y., & Murata, N. (2014). Revised scheme for the mechanism of photoinhibition and its application to enhance the abiotic stress tolerance of the photosynthetic machinery. Applied Microbiology and Biotechnology, 98, 8777–8796.
Foyer, C. H., & Noctor, G. (2016). Stress-triggered redox signalling: What’s in pROSpect? Plant, Cell and Environment, 39, 951–964.
Keeling, P. J. (2013). The number, speed, and impact of plastid endosymbioses in eukaryotic evolution. Annual Review of Plant Biology, 64, 583–607.
Stiller, J. W., Schreiber, J., Yue, J., Guo, H., Ding, Q., & Huang, J. (2014). The evolution of photosynthesis in chromist algae through serial endosymbioses. Nature Communications, 5, 1–7.
Gould, S. B., Waller, R. F., & McFadden, G. I. (2008). Plastid evolution. Annual Review of Plant Biology, 59, 491–517.
Dorrell, R. G., Gile, G., McCallum, G., Méheust, R., Bapteste, E. P., Klinger, C. M., Brillet-Guéguen, L., Freeman, K. D., Richter, D. J., & Bowler, C. (2017). Chimeric origins of ochrophytes and haptophytes revealed through an ancient plastid proteome. eLife, 6, 1–45.
Kovács, L., Ayaydin, F., Kálai, T., Tandori, J., Kós, P. B., & Hideg, É. (2014). Assessing the applicability of singlet oxygen photosensitizers in leaf studies. Photochemistry and Photobiology, 90, 129–136.
Takagi, D., Takumi, S., Hashiguchi, M., Sejima, T., & Miyake, C. (2016). Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiology, 171, 1626–1634.
Okada, K., Ikeuchi, M., Yamamoto, N., Ono, T. A., & Miyao, M. (1996). Selective and specific cleavage of the D1 and D2 proteins of Photosystem II by exposure to singlet oxygen: Factors responsible for the susceptibility to cleavage of the proteins. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1274, 73–79.
Fischer, B. B., Hideg, É., & Krieger-Liszkay, A. (2013). Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxidants Redox Signal., 18, 2145–2162.
Ben Sheleg, A., Novoplansky, N., & Vonshak, A. (2019). Can Rose Bengal resilience be used as a marker for photosynthetic resilience of Nannochloropsis oceanica strains in excess light environments? (p. 41). Elsevier.
Ben-Sheleg, A., & Vonshak, A. (2022). Tolerance to exogenously added ROS examined for correlation with enhanced specific growth rates of Arthrospira platensis. Journal of Applied Phycology. https://doi.org/10.1007/s10811-022-02688-0
Dall’Osto, L., Cazzaniga, S., Guardini, Z., Barera, S., Benedetti, M., Mannino, G., Maffei, M. E., & Bassi, R. (2019). Combined resistance to oxidative stress and reduced antenna size enhance light-to-biomass conversion efficiency in Chlorella vulgaris cultures. Biotechnology for Biofuels, 12, 1–17.
Ben-sheleg, A., Khozin-godberg, I., Yaakov, B., & Vonshak, A. (2021). Characterization of Nannochloropsis oceanica rose Bengal mutants sheds light on acclimation mechanisms to high light when grown in low temperature a barrier to realizing nannochloropsis oceanica’s potential. PCP, 62, 1478–1493.
Seely, G. R., Duncan, M. J., & Vidaver, W. E. (1972). Preparative and analytical extraction of pigments from brown algae with dimethyl sulfoxide. Marine Biology, 12, 184–188.
Monod, J., Wyman, J., & Changeux, J. P. (1965). On the nature of allosteric transitions: A plausible model. Journal of Molecular Biology, 12, 88–118.
Strasser, R. J., Srivastava, A., & Tsimilli-Michael, M. (1999). In M. Yunus, U. Pathre, & P. Mohanty (Eds.), Probing photosynthesis mechanisms, regulation and adaptation (pp. 445–483). Taylor & Francis.
Stirbet, A., Lazár, D., Kromdijk, J., & Govindjee,. (2018). Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica, 56, 1–19.
Babbs, C. F., Pham, J. A., & Coolbaugh, R. C. (1989). Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiology, 90, 1267–1270.
Cui, F., Brosché, M., Shapiguzov, A., He, X. Q., Vainonen, J. P., Leppälä, J., Trotta, A., Kangasjärvi, S., Salojärvi, J., Kangasjärvi, J., & Overmyer, K. (2019). Interaction of methyl viologen-induced chloroplast and mitochondrial signalling in Arabidopsis. Free Radical Biology & Medicine, 134, 555–566.
Nama, S., Madireddi, S. K., Yadav, R. M., & Subramanyam, R. (2019). Non-photochemical quenching-dependent acclimation and thylakoid organization of Chlamydomonas reinhardtii to high light stress. Photosynthesis Research, 139, 387–400.
Cao, S., Zhang, X., Xu, D., Fan, X., Mou, S., Wang, Y., Ye, N., & Wang, W. (2013). A transthylakoid proton gradient and inhibitors induce a non-photochemical fluorescence quenching in unicellular algae Nannochloropsis sp. FEBS Letters, 587, 1310–1315.
Białasek, M., Górecka, M., Mittler, R., & Karpiński, S. (2017). Evidence for the involvement of electrical, calcium and ROS signaling in the systemic regulation of non-photochemical quenching and photosynthesis. Plant and Cell Physiology, 58, 207–215.
Dong, Y. L., Jiang, T., Xia, W., Dong, H. P., Lu, S. H., & Cui, L. (2015). Light harvesting proteins regulate non-photochemical fluorescence quenching in the marine diatom Thalassiosira pseudonana. Algal Research, 12, 300–307.
Miyake, C. (2010). Alternative electron flows (water-water cycle and cyclic electron flow around PSI) in photosynthesis: Molecular mechanisms and physiological functions. Plant and Cell Physiology, 51, 1951–1963.
Zavřel, T., Szabó, M., Tamburic, B., Evenhuis, C., Kuzhiumparambil, U., Literáková, P., Larkum, A. W. D., Raven, J. A., Červený, J., & Ralph, P. J. (2018). Effect of carbon limitation on photosynthetic electron transport in Nannochloropsis oculata. Journal of Photochemistry and Photobiology, B, 181, 31–43.
Sétif, P. (2015). Electron-transfer kinetics in cyanobacterial cells: Methyl viologen is a poor inhibitor of linear electron flow. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1847, 212–222.
Chagas, R. M., Silveira, J. A. G., Ribeiro, R. V., Vitorello, V. A., & Carrer, H. (2008). Photochemical damage and comparative performance of superoxide dismutase and ascorbate peroxidase in sugarcane leaves exposed to paraquat-induced oxidative stress. Pesticide Biochemistry and Physiology, 90, 181–188.
Martin, R. E., Thomas, D. J., Tucker, D. E., & Herbert, S. K. (1997). The effects of photooxidative stress on photosystem I measured in vivo in Chlamydomonas. Plant, Cell and Environment, 20, 1451–1461.
Collén, J., & Davison, I. R. (1999). Stress tolerance and reactive oxygen metabolism in the intertidal red seaweeds Mastocarpus stellatus and Chondrus crispus. Plant, Cell and Environment, 22, 1143–1151.
Gutiérrez, J., González-Pérez, S., García-García, F., Daly, C. T., Lorenzo, Ó., Revuelta, J. L., McCabe, P. F., & Arellano, J. B. (2014). Programmed cell death activated by Rose Bengal in Arabidopsis thaliana cell suspension cultures requires functional chloroplasts. Journal of Experimental Botany, 65, 3081–3095.
Guo, L., Liang, S., Zhang, Z., Liu, H., Wang, S., Pan, K., Xu, J., Ren, X., Pei, S., & Yang, G. (2019). Genome assembly of Nannochloropsis oceanica provides evidence of host nucleus overthrow by the symbiont nucleus during speciation. Communications Biology, 2, 1–12.
Koh, E., Carmieli, R., Mor, A., & Fluhr, R. (2016). Singlet oxygen-induced membrane disruption and serpin-protease balance in vacuolar-driven cell death. Plant Physiology, 171, 1616–1625.
Dogra, V., & Kim, C. (2020). Singlet oxygen metabolism: From genesis to signaling. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2019.01640
Ramel, F., Birtic, S., Ginies, C., Soubigou-Taconnat, L., Triantaphylidès, C., & Havaux, M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proceedings of the National Academy of Sciences, 109, 5535–5540.
Wang, L., Leister, D., Guan, L., Zheng, Y., Schneider, K., Lehmann, M., Apel, K., & Kleine, T. (2020). The Arabidopsis SAFEGUARD1 suppresses singlet oxygen-induced stress responses by protecting grana margins. Proceedings of the National Academy of Sciences, 117, 6918–6927.
Wang, L., Kim, C., Xu, X., Piskurewicz, U., Dogra, V., Singh, S., Mahler, H., & Apel, K. (2016). Singlet oxygen- and EXECUTER1-mediated signaling is initiated in grana margins and depends on the protease FtsH2. PNAS, 113, E3792–E3800.
Fischer, B. B., Krieger-Liszkay, A., Hideg, É., Šnyrychová, I., Wiesendanger, M., & Eggen, R. I. L. (2007). Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Letters, 581, 5555–5560.
Fischer, B. B., Ledford, H. K., Wakao, S., Huang, S. Y. G., Casero, D., Pellegrini, M., Merchant, S. S., Koller, A., Eggen, R. I. L., & Niyogi, K. K. (2012). Singlet oxygen resistant 1 links reactive electrophile signaling to singlet oxygen acclimation in Chlamydomonas reinhardtii. PNAS, 109, E1302–E1311.
Acknowledgements
This research was conducted without specific grants from any public or private agency. The authors would like to thank Samara Bel for her editing of this work. The authors would like to thank the reviewers of this work for their meticulous work and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of both authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben-Sheleg, A., Vonshak, A. Photoacclimation of photosystem II photochemistry induced by rose Bengal and methyl viologen in Nannochloropsis oceanica. Photochem Photobiol Sci 21, 2205–2215 (2022). https://doi.org/10.1007/s43630-022-00289-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-022-00289-x