Abstract
The initial objective of our work was to synthesize a series of 2-amino-4H-pyran-3-carbonitriles to be tested for their antifungal activities against economically relevant phytopathogenic fungi. Fourteen compounds were prepared in up to 94% yield and shown percentages of Botrytis cinerea inhibition above 70%. Despite the promising biological results, we observed that stock solutions prepared for biological tests showed color changing when kept for a few days on the laboratory bench, under room conditions, illuminated by common LED daylight tubes (4500–6000 k). This prompted us to investigate the possible photo-induced degradation of our compounds. FT-IR ATR experiments evidenced variations in the expected bands for functional of -amino-4H-pyran-3-carbonitriles stored under LED daylight. Following, HPLC–UV analysis showed reductions in the intensity of chromatographic peaks of 2-amino-4H-pyran-3-carbonitriles, and but not for solutions kept in the dark. A solution of (E)-2-amino-8-(4-nitrobenzylidene)-4-(4-nitrophenyl)-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile underwent 84.4% of conversion after 72 h of exposure to continuous LED daylight in a BOD chamber, and the reaction product was isolated in 36% yield and characterized as (E)-7-cyano-5-(4-nitrobenzylidene)-8-(4-nitrophenyl)bicyclo[4.2.0]oct-1(6)-ene-7-carboxamide (7*). Despite freshly prepared solutions of 2-amino-4H-pyran-3-carbonitriles produced antifungal activities, these solutions lost biological activity when left on the bench for a week. Besides, compound 7* formed from photo-induced degradation of 7 also showed no antifungal activity. With this, we hope to bring two contributions: (1) production of cyclobutenes through photochemical reactions of 2-amino-4H-pyran-3-carbonitriles can be carried out through exposure to simple white LED daylight; (2) biological applications of such 2-amino-4H-pyran-3-carbonitriles may be impaired by their poor photostability.
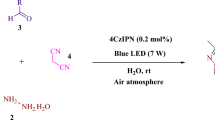
Graphic abstract









Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Kumar, D., Sharma, P., Singh, H., Nepali, K., Gupta, G. K., Jain, S. K., & Ntie-Kang, F. (2017). The value of pyrans as anticancer scaffolds in medicinal chemistry. RSC Advances, 7, 36977–36999.
Verma, A. K., Bishnoi, A., Fatma, S., Srivastava, A., & Singh, V. (2016). A rapid and efficient route to synthesis of 2-amino-4H-pyran-3-carbonitrile derivatives in the presence of L-proline and their antimicrobial activity. Der Pharma Chemica, 8, 380–391.
Wang, D.-C., Xie, Y.-M., Fan, C., Yao, S., & Song, H. (2014). Efficient and mild cyclization procedures for the synthesis of novel 2-amino-4H-pyran derivatives with potential antitumor activity. Chinese Chemical Letters, 25, 1011–1013.
Rao, N. K., Rao, T. N., Parvatamma, B., Devi, K. P., & Setty, S. C. (2018). Multi component one pot synthesis and characterization of derivatives of 2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile and study of anti-microbial activity. Bulletin of the Chemical Society of Ethiopia, 32, 133.
Bhosle, M. R., Wahul, D. B., Bondle, G. M., Sarkate, A., & Tiwari, S. V. (2018). An efficient multicomponent synthesis and in vitro anticancer activity of dihydropyranochromene and chromenopyrimidine-2,5-diones. Synthetic Communications, 48, 2046–2060.
Ahmed, M. H., El-Hashash, M. A., Marzouk, M. I., & El-Naggar, A. M. (2019). Design, synthesis, and biological evaluation of novel pyrazole, oxazole, and pyridine derivatives as potential anticancer agents using mixed chalcone. Journal of Heterocyclic Chemistry, 56, 114–123.
Bayomi, S. M., El-Kashef, H. A., El-Ashmawy, M. B., Nasr, M. N. A., El-Sherbeny, M. A., Badria, F. A., Abou-zeid, L. A., Ghaly, M. A., & Abdel-Aziz, N. I. (2013). Synthesis and biological evaluation of new curcumin derivatives as antioxidant and antitumor agents. Medicinal Chemistry Research, 22, 1147–1162.
Al-Omar, M. A., Youssef, K. M., El-Sherbeny, M. A., Awadalla, S. A. A., & El-Subbagh, H. I. (2005). Synthesis and in vitro antioxidant activity of some new fused pyridine analogs. Archiv der Pharmazie (Weinheim), 338, 175–180.
Soylem, A. E., Assy, G. M., & Morsi, M. G. (2016). Heteroannelation of cyclic ketones: Synthesis, characterization and antitumor evaluation of some condensed azine derivatives. Acta Chimica Slovenica, 63, 609–618.
Wen-Sun, C., Wang, J., Cheng-Pang, C., Li, J., & Xiao, D. (2013). Synthesis and anti-leukemia evaluation of tetrahydro-4H-pyrano[3,2-c]pyridines and corresponding anti-CD14 monoclonal antibody conjugates. Chemical Research in Chinese Universities, 29, 1104–1109.
Kumar, R. R., Perumal, S., Senthilkumar, P., Yogeeswari, P., & Sriram, D. (2007). An atom efficient, solvent-free, green synthesis and antimycobacterial evaluation of 2-amino-6-methyl-4-aryl-8-[(E)-arylmethylidene]-5,6,7,8-tetrahydro-4H-pyrano[3,2-c]pyridine-3-carbonitriles. Bioorganic & Medicinal Chemistry Letters, 17, 6459–6462.
Patil, S. A. C., Patil, S. R., Patil, V. M., Patil, S. V., Jachak, M. N., & Desai, A. (2015). Synthesis of pyrano[2,3-d]pyridine, pyrazolo[3,4-b]pyridine derivatives by microwave irradiation and study of their insecticidal activity. Journal of Chemical and Pharmaceutical Research, 7, 476–482.
Witschel, M., Stelzer, F., Hutzler, J., Qu, T., Mietzner, T., Kreuz, K., Grobmann, K., Aponte, R., Hoffken, H.W., Calo, F., Ehrhardt, T., Simon, A., Parra Rapado, L. (2016). Pyrazolopyrans having herbicidal and pharmaceutical properties, PCT Int Appl WO 2013182472 A1.
Sun, W., Jiang, Y., Yan, H., & Song, X. (2015). Synthesis and photoreaction of 2-amino-3-cyano-4-aryl-4H-pyrans. Australian Journal of Chemistry, 68, 273.
Armesto, D., Horspool, W. M., Martin, N., Ramos, A., & Seoane, C. (1989). Synthesis of cyclobutenes by the novel photochemical ring contraction of 4-substituted 2-amino-3,5-dicyano-6-phenyl-4H-pyrans. Journal of Organic Chemistry, 54, 3069–3072.
Armesto, D., Albert, A., Cano, F. H., Martín, N., Ramos, A., Rodriguez, M., Segura, J. L., & Seoane, C. (1997). A study on the scope of the photochemical ring contraction of substituted 2-amino-3-cyano-4H-pyrans to cyclobutenes: Crystal structure of 3-carbamoyl-3-cyano-1-ethoxycarbonyl-4-isopropyl-2-phenylcyclobutene. Journal of the Chemical Society, Perkin Transactions, 1, 3401–3406.
Armesto, D., Horspool, W. M., Martin, N., Ramos, A., & Seoane, C. (1987). A novel photochemical ring contraction of 4H-Pyrans. A new route to selectively substituted cyclobutenes. Journal of the Chemical Society, Chemical Communications, 1987, 1231–1232.
Karimi-Jaberi, Z., & Pooladian, B. (2012). A facile synthesis of new 2-amino-4 h-pyran-3-carbonitriles by a one-pot reaction of α, α-Bis(arylidene) cycloalkanones and malononitrile in the presence of K2CO3. The Scientific World Journal, 2012, 3–7.
Cimmino, A., Nocera, P., Linaldeddu, B. T., Masi, M., Gorecki, M., Pescitelli, G., Montecchio, L., Maddau, L., & Evidente, A. (2018). Phytotoxic metabolites produced by Diaporthella cryptica, the causal agent of Hazelnut Branch Canker. Journal of Agriculture and Food Chemistry, 66, 3435–3442.
Anastas, P., & Eghbali, N. (2010). Green chemistry: Principles and practice. Chemical Society Reviews, 39, 301–312.
Jin, T. S., Bin Liu, L., Zhao, Y., & Li, T. S. (2005). Clean, one-pot synthesis of 4H-pyran derivatives catalyzed by hexadecyltrimethyl ammonium bromide in aqueous media. Synthetic Communications, 35, 1859–1863.
Tiwari, J., Saquib, M., Singh, S., Tufail, F., Singh, M., Singh, J., & Singh, J. (2016). Visible light promoted synthesis of dihydropyrano[2,3-c] chromenes via a multicomponent-tandem strategy under solvent and catalyst free conditions. Green Chemistry, 18, 3221–3231.
Lazzaroni, S., Dondi, D., Mezzetti, A., & Protti, S. (2018). Role of solute-solvent hydrogen bonds on the ground state and the excited state proton transfer in 3-hydroxyflavone. A systematic spectrophotometric study. Photochemical and Photobiological Sciences, 17, 923–933.
Letourneau, D. R., Gill, C. G., & Krogh, E. T. (2015). Photosensitized degradation kinetics of trace halogenated contaminants in natural waters using membrane introduction mass spectrometry as an in situ reaction monitor. Photochemical & Photobiological Sciences, 14, 2108–2118.
Shvydkiv, O., Yavorskyy, A., Nolan, K., Youssef, A., Riguet, E., Hoffmann, N., & Oelgemöller, M. (2010). Photosensitized addition of isopropanol to furanones in a 365 nm UV-LED microchip. Photochemical & Photobiological Sciences, 9, 1601.
Acknowledgements
We thanks the Brazilian agencies FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), and FINEP (Financiadora de Estudos e Projetos) for financial support.
Funding
This work was supported by the following Brazilian agencies: Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) (Grant nos. 459271/2014-8 and 438712/2018-8) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (Grant no. APQ-02789-14, APQ-01577-17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Silva, U.P., de Sousa, B.L., Ferreira, B.W. et al. Daylight LED promotes photochemical ring contraction of 2-amine-4H-pyran-3-carbonitriles with consequent loss of their antifungal activity. Photochem Photobiol Sci 20, 1309–1321 (2021). https://doi.org/10.1007/s43630-021-00108-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-021-00108-9