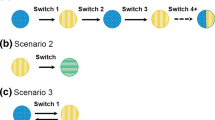
Abstract
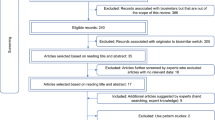
To date, numerous biosimilars are available in Europe and the practice of switching between originator and biosimilar or between two different biosimilars has become very widespread. However, multiple switching has not been adequately studied. The aim of this study is to conduct a literature review to assess the effectiveness and safety of multiple switches. All PubMed articles involving multiple switches from originator to biosimilars or between different biosimilars were considered. The relevant data on effectiveness and safety were extracted from these studies and the results were reported through descriptive analysis. Fifteen studies were considered, of which 11 were observational and 4 clinical trials. Inflammatory bowel disease and psoriasis were the most studied diseases. All studies reported that the effectiveness and safety in patients whose treatment involved multiple switches, was comparable to patients whose treatment involved a single or no switch at all. Some therapeutic fields such as oncology and renal insufficiency were not represented at all in the multiple switch studies. New evidence is desperately needed and should be made available to the scientific community and decision-makers.

Similar content being viewed by others
References
DeVries JH, Gough SC, Kiljanski J, Heinemann L. Biosimilar insulins: a European perspective. Diabetes Obes Metab. 2015;17(5):445–51.
Saenger P. Biosimilar growth hormone. Indian J Pediatr. 2012;79(1):92–8.
Azevedo A, Bettencourt A, Selores M, Torres T. Biosimilar agents for psoriasis treatment: the perspective of Portuguese patients. Acta Med Port. 2018;31(9):496–500.
Lyman GH. New biosimilar approvals for myeloid growth factors and anemia. J Natl Compr Cancer Netw. 2019;17(5.5):622–4.
Renwick MJ, Smolina K, Gladstone EJ, Weymann D, Morgan SG. Postmarket policy considerations for biosimilar oncology drugs. Lancet Oncol. 2016;17(1):e31-8.
Smolen JS, Goncalves J, Quinn M, Benedetti F, Lee JY. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open. 2019;5(1):e000900.
Buchner AM, Schneider Y, Lichtenstein GR. Biosimilars in Inflammatory bowel disease. Am J Gastroenterol. 2021;116(1):45–56.
Dutta B, Huys I, Vulto AG, Simoens S. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020;34(2):159–70.
Glintborg B, Sorensen J, Hetland ML. Does a mandatory non-medical switch from originator to biosimilar infliximab lead to increased use of outpatient healthcare resources? A register-based study in patients with inflammatory arthritis. RMD Open. 2018;4(2):e000710.
McKinnon RA, Cook M, Liauw W, et al. Biosimilarity and interchangeability: principles and evidence: a systematic review. BioDrugs. 2018;32(1):27–52.
May MB, Taucher KD, Vogel WH. Practical considerations for integrating biosimilars into clinical practice. J Adv Pract Oncol. 2021;12(4):431–8.
Rifkin RM, Peck SR. Biosimilars: implications for clinical practice. J Oncol Pract. 2017;13(9_suppl):24s–31s.
Bridges SL Jr, White DW, Worthing AB, et al. The science behind biosimilars: entering a new era of biologic therapy. Arthritis Rheumatol. 2018;70(3):334–44.
Griffith N, McBride A, Stevenson JG, Green L. Formulary selection criteria for biosimilars: considerations for US health-system pharmacists. Hosp Pharm. 2014;49(9):813–25.
Harsanyi A, Csanadi M, Marky K, Vincziczki AZ, Kalo Z, Inotai A. Influence of biosimilar infliximab launch on the utilization pattern of biological medicines: the case of Hungary. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):653–9.
Blackwell K, Gascon P, Krendyukov A, Gattu S, Li Y, Harbeck N. Safety and efficacy of alternating treatment with EP2006, a filgrastim biosimilar, and reference filgrastim: a phase III, randomised, double-blind clinical study in the prevention of severe neutropenia in patients with breast cancer receiving myelosuppressive chemotherapy. Ann Oncol. 2018;29(1):244–9.
Genovese MC, Kellner H, Arai Y, Muniz R, Alten R. Long-term safety, immunogenicity and efficacy comparing FKB327 with the adalimumab reference product in patients with active rheumatoid arthritis: data from randomised double-blind and open-label extension studies. RMD Open. 2020;6(1):e000987.
Gisondi P, Virga C, Piaserico S, et al. Switching from one infliximab biosimilar (CT-P13) to another infliximab biosimilar (SB2) in patients with chronic plaque psoriasis. Br J Dermatol. 2020;183(2):397–8.
Griffiths CEM, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–38.
Hanzel J, Jansen JM, Ter Steege RWF, Gecse KB, D’Haens GR. Multiple switches from the originator infliximab to biosimilars is effective and safe in inflammatory bowel disease: a prospective multicenter cohort study. Inflamm Bowel Dis. 2021. https://doi.org/10.1093/ibd/izab099.
Lovero R, Losurdo G, La Fortezza RF, et al. Safety and efficacy of switching from infliximab biosimilar CT-P13 to infliximab biosimilar SB2 in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2021;32(2):201–7.
Mazza S, Piazza OSN, Conforti FS, et al. Safety and clinical efficacy of the double switch from originator infliximab to biosimilars CT-P13 and SB2 in patients with inflammatory bowel diseases (SCESICS): a multicenter cohort study. Clin Transl Sci. 2022;15(1):172–81.
Piaserico S, Conti A, Messina F, et al. Cross-switch from etanercept originator to biosimilar SB4 and to GP2015 in patients with chronic plaque psoriasis. BioDrugs. 2021;35(4):469–71.
Ribaldone DG, Tribocco E, Rosso C, et al. Switching from biosimilar to biosimilar adalimumab, including multiple switching, in Crohn’s disease: a prospective study. J Clin Med. 2021;10(15):387.
Trystram N, Abitbol V, Tannoury J, et al. Outcomes after double switching from originator Infliximab to biosimilar CT-P13 and biosimilar SB2 in patients with inflammatory bowel disease: a 12-month prospective cohort study. Aliment Pharmacol Ther. 2021;53(8):887–99.
Macaluso FS, Fries W, Viola A, et al. The SPOSIB SB2 Sicilian cohort: safety and effectiveness of infliximab biosimilar SB2 in inflammatory bowel diseases including multiple switches. Inflamm Bowel Dis. 2021;27(2):182–9.
Blauvelt A, Lacour JP, Fowler JF Jr, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623–31.
Lauret A, Molto A, Abitbol V, et al. Effects of successive switches to different biosimilars infliximab on immunogenicity in chronic inflammatory diseases in daily clinical practice. Semin Arthritis Rheum. 2020;50(6):1449–56.
Ilias A, Szanto K, Gonczi L, et al. Outcomes of patients with inflammatory bowel diseases switched from maintenance therapy with a biosimilar to Remicade. Clin Gastroenterol Hepatol. 2019;17(12):2506-13 e2502.
Commission EMAatE. Biosimilars in the EU - Information guide for healthcare professionals. 2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed 27 June 2022.
Kurki P. No need for systematic switch studies to demonstrate interchangeability of biosimilars. GaBI J. 2022;11(1):5–6.
Gherghescu I, Delgado-Charro MB. The biosimilar landscape: an overview of regulatory approvals by the EMA and FDA. Pharmaceutics. 2020;13(1):48.
Agency EM. European public assessment reports (EPAR). 2022. https://www.ema.europa.eu/en/medicines/download-medicine-data#european-public-assessment-reports-(epar)-section. Accessed 7 Apr 2022.
Hung CC, Tsai IC, Hsu CY, Lin HC. Clinical outcomes of neoadjuvant therapy in human epidermal growth factor receptor 2 breast cancer patients: a single-center retrospective study. J Clin Med. 2022;11(5):1434.
Oza AM, Dubois F, Hegg R, et al. A long-term extension study of bevacizumab in patients with solid tumors. Oncologist. 2021;26(12):e2254-64.
Sun D, Andayani TM, Altyar A, MacDonald K, Abraham I. Potential cost savings from chemotherapy-induced febrile neutropenia with biosimilar filgrastim and expanded access to targeted antineoplastic treatment across the European Union G5 countries: a simulation study. Clin Ther. 2015;37(4):842–57.
Agency EM. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf. Accessed 8 Apr 2022.
Feagan BG, Marabani M, Wu JJ, Faccin F, Spronk C, Castaneda-Hernandez G. The challenges of switching therapies in an evolving multiple biosimilars landscape: a narrative review of current evidence. Adv Ther. 2020;37(11):4491–518.
Barbier L, Mbuaki A, Simoens S, Declerck P, Vulto AG, Huys I. Regulatory information and guidance on biosimilars and their use across europe: a call for strengthened one voice messaging. Front Med (Lausanne). 2022;9:820755.
Agency EM. Biosimilars in the EU - Information guide for healthcare professionals. 2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Updated 2 Oct 2019.
US Food and Drug Administration. Purple Book - Database of Licensed Biological Products. 2022. https://purplebooksearch.fda.gov. Updated 1 Sep 2022. Accessed 25 Sep 2022.
US Food and Drug Administration. Considerations in demonstrating interchangeability with a reference product. Guidance for industry. 2019. https://www.fda.gov/media/124907/download. Updated May 2019. Accessed 25 Sep 2022.
McKinley L, Kelton JM, Popovian R. Sowing confusion in the field: the interchangeable use of biosimilar terminology. Curr Med Res Opin. 2019;35(4):619–21.
Alvarez DF, Wolbink G, Cronenberger C, Orazem J, Kay J. Interchangeability of biosimilars: what level of clinical evidence is needed to support the interchangeability designation in the United States? BioDrugs. 2020;34(6):723–32.
Administration UFaD. FDA Approves Cyltezo, the First Interchangeable Biosimilar to Humira. 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-cyltezo-first-interchangeable-biosimilar-humira. Updated 18 Oct 2021. Accessed 25 Sep 2022.
(AIFA) IMA. Second Position Paper AIFA sui Farmaci Biosimilari. https://www.aifa.gov.it/documents/20142/241044/2_Position-Paper-AIFA-Farmaci-biosimilari.pdf. Accessed 9 Apr 2022.
Addis A. Biosimilars: Italian medicines agency takes position. Recenti Prog Med. 2018;109(4):214–5.
Vogler S, Schneider P, Zuba M, Busse R, Panteli D. Policies to Encourage the use of biosimilars in European countries and their potential impact on pharmaceutical expenditure. Front Pharmacol. 2021;12:625296.
Michael Sarshad RC, Pitts PJ, Vanderpuye-Orgle J. The need for distinct nomenclature for originator and biosimilar products. GaBI J. 2018;7(4):152–7.
Funding
The authors received no funding.
Author information
Authors and Affiliations
Contributions
RL made contribution to the conception and design, acquisition of data, analysis and interpretation of data, and article writing. PA, AZ and FS made contribution to the acquisition of data, analysis and interpretation of data, article writing and gave the final approval. All the authors declare that the opinions expressed are of a personal nature and do not in any way commit the responsibility of the Administrations to which they belong.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lasala, R., Abrate, P., Zovi, A. et al. Safety and Effectiveness of Multiple Switching Between Originators and Biosimilars: Literature Review and Status Report on Interchangeability. Ther Innov Regul Sci 57, 352–364 (2023). https://doi.org/10.1007/s43441-022-00473-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-022-00473-2