Abstract
Background
The last two decades have witnessed the vigorous development of targeted cancer drugs and the potent therapeutic effects of these drugs have been validated by various true and surrogate end points. Overall survival (OS) and progression-free survival (PFS) outcomes were two important end points used in targeted cancer drugs clinical trials but investigation on which was rare as a consequence of inherent heterogeneity and complexity. Here, we present the review and analysis of OS and PFS outcomes of all targeted cancer drugs approved by the U.S. Food and Drug Administration (FDA) through December 31, 2019, in the setting of clinical studies.
Methods
The targeted cancer drug directory was accessed via NCI website. The survival outcomes of those drugs in the setting of clinical trials were collected from publicly available Package Inserts and ClinicalTrials.gov. Median overall (OS) and progression-free survival (PFS) outcomes were summarized for targeted cancer drugs that were evaluated as monotherapies in clinical trials, contributions of targeted drugs to combination therapies in terms of survival benefit were analyzed, survival outcomes of FDA-approved first-line targeted therapies in different cancers were investigated, and the correlation between the absolute OS gain (ΔOS) and PFS gain (ΔPFS) as well as the hazard ratios for PFS (HRPFS) and OS (HROS) between comparative arms of available randomized clinical trials was evaluated.
Results
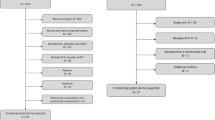
A total of 223 indications for 126 targeted cancer drugs have been approved by the FDA through December 31, 2019, among which 28 indications of 23 drugs have been approved for first-line therapies. Eighty indications for leukemia, lymphoma, and rare cancer types without survival data were excluded in the investigation of survival outcomes. For the remaining 143 indications, 99 were approved as monotherapies and 72 were approved as combination therapies. Among monotherapy subset, 18 of 72 (25%) indications with available OS outcomes have maximum median OS less than 12 months, 55 of 72 (76%) drug indications have maximum median OS less than 24 months, and 67 of 72 (93%) have maximum median OS less than 36 months. Regarding PFS, 38 of 88 (43%) drug indications have maximum median PFS less than 6 months, 69 of 88 (78%) drug indications have maximum median PFS less than 12 months, and 84 of 88 (95%) drug indications have maximum median PFS less than 24 months. The addition of targeted drugs to combination regimens under FDA’s approval provided notable survival benefit, but the median value of OS gain rate of the available combination regimens was lower than that of PFS. The univariate Spearman Rank correlation coefficient suggested a significant positive correlation between ΔOS and ΔPFS (R = 0.62) but a weak correlation between HROS and HRPFS (R = 0.11) in 46 randomized clinical trials that investigated contributions of targeted cancer drugs to combination therapies with available data. A moderate correlation between ΔOS and ΔPFS (R = 0.43) and a rather weak correlation between HROS and HRPFS (R = 0.07) were also found in other randomized clinical trials that included in our study with available data.
Conclusions
The survival benefit provided by targeted cancer drugs varied a lot among cancer types. Though targeted cancer drugs showed improvements in both OS and PFS in approved combination regimens, the median value of OS gain rate was lower than that of PFS. As only weak correlation was found between HRPFS and HROS, the evidence supporting the use of PFS as a surrogate to OS in the setting of targeted cancer drugs might also be limited.



Similar content being viewed by others
References
National Cancer Institute (NCI). Targeted therapy to treat cancer. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies. Accessed 31 Dec 2019.
Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–96.
National Cancer Institute (NCI). What targeted therapies have been approved for specific types of cancer? https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies. Accessed 31 Dec 2019.
Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol. 2003;21(7):1404–11.
Lesko LJ, Atkinson AJ. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu Rev Pharmacol. 2001;41:347–66.
Clinical trial endpoints for the approval of cancer drugs and biologics, guidance for industry, December 2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents. Accessed 31 Dec 2019.
Pazdur R. Endpoints for assessing drug activity in clinical trials. Oncologist. 2008;13:19–21.
Naci H, Smalley KR, Kesselheim AS. Characteristics of preapproval and postapproval studies for drugs granted accelerated approval by the US Food and Drug Administration. JAMA. 2017;318(7):626–36.
Smith BD, DeZern AE, Bastian AW, Durie BGM. Meaningful endpoints for therapies approved for hematologic malignancies. Cancer Am Cancer Soc. 2017;123(10):1689–94.
Svensson S, Menkes DB, Lexchin J. Surrogate outcomes in clinical trials a cautionary tale. JAMA Intern Med. 2013;173(8):611–2.
Prasad V, Kim C, Burotto M, Vandross A. The strength of association between surrogate end points and survival in oncology: a systematic review of trial-level meta-analyses. JAMA Intern Med. 2015;175(8):1389–98.
Haslam A, Hey SP, Gill J, Prasad V. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur J Cancer. 2019;106:196–211.
Blumenthal GM, Karuri SW, Zhang H, Zhang LJ, Khozin S, Kazandjian D, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33(9):1008+.
Shi Q, Sargent DJ. Meta-analysis for the evaluation of surrogate endpoints in cancer clinical trials. Int J Clin Oncol. 2009;14(2):102–11.
Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005–2012. JAMA. 2014;311(4):368–77.
Redberg RF. Faster drug approvals are not always better and can be worse. JAMA Intern Med. 2015;175(8):1398–1398.
Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365(2):e3.
Ocana A, Amir E, Vera F, Eisenhauer EA, Tannock IF. Addition of bevacizumab to chemotherapy for treatment of solid tumors: similar results but different conclusions. J Clin Oncol. 2011;29(3):254–6.
RStudio Team. RStudio: Integrated Development for R. Boston: RStudio, PBC; 2020. http://www.rstudio.com/.
Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–85.
Lakdawalla DN, Chou JW, Linthicum MT, MacEwan JP, Zhang J, Goldman DP. Evaluating expected costs and benefits of granting access to new treatments on the basis of progression-free survival in non-small-cell lung cancer. JAMA Oncol. 2015;1(2):196–202.
Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16.
Zahorowska B, Crowe PJ, Yang JL. Combined therapies for cancer: a review of EGFR-targeted monotherapy and combination treatment with other drugs. J Cancer Res Clin. 2009;135(9):1137–48.
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14.
Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(8):e198890.
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Author information
Authors and Affiliations
Contributions
LMS and QH conceived and designed the study. QH evaluated the data and wrote the manuscript. QL and FZL devised the study and contributed to the manuscript. KIK and LMS reviewed and edited the manuscript. The authors would like to acknowledge LX for the assistance with statistical analysis for this study. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, Q., Li, Q., Lv, F. et al. A Survey of Survival Outcomes for Targeted Cancer Drugs Approved by the US Food and Drug Administration. Ther Innov Regul Sci 55, 676–684 (2021). https://doi.org/10.1007/s43441-021-00264-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-021-00264-1