Abstract
Background
Graves’ orbitopathy (GO) is an autoimmune disorder of the orbit and retro-ocular tissues and the primary extrathyroidal manifestation of Graves’ disease. In moderate-to-severe and active GO iv glucocorticoids (GCs) are recommended as first-line treatment. The aim was to assess the safety profile of methylprednisolone administered intravenously for three consecutive days at 1 g in patients with active, moderate-to-severe or sight-threatening Graves’ orbitopathy.
Methods
We retrospectively evaluated 161 medical records of patients with GO treated with high-dose systemic GCs in the Department of Endocrinology, Metabolic Disorders, and Internal Medicine in Poznań between 2014 and 2021. Clinical data included age, gender, laboratory results, activity and severity of GO, smoking status, disease duration, and presented side effects.
Results
The presence of mild side effects was observed during 114 (71%) hospitalizations. The most common complications were hyperglycemia (n = 95) and elevated aminotransferases (n = 31). Increased levels of aminotransferases were more likely observed in smokers and GO duration above 12 months. Based on the multivariate logistic regression, higher TRAb and CAS values were significantly associated with lower odds of hyperglycemia. In turn, the increased odds of elevated aminotransferases were significantly correlated with higher initial ALT levels, female gender, and GO duration above 12 months. In addition, the multidimensional correspondence analysis (MPA) showed that GO patients who declared smoking and had not l-ornithine l-aspartate applied demonstrated a higher probability of elevated aminotransferases.
Conclusions
Active GO treatment with high-dose systemic GCs is not associated with serious side effects. Hyperglycemia is the most common steroid-induced complication.
Similar content being viewed by others
Introduction
Graves’ orbitopathy (GO), also called Graves’ ophthalmopathy, thyroid-associated ophthalmopathy (TAO), or thyroid eye disease (TED), is an autoimmune disorder of the orbit and retrobulbar tissues, representing the major extrathyroidal manifestation of Graves’ disease (GD) [1,2,3]. Although GO is most frequently associated with hyperthyroidism, it may rarely occur in euthyroid or hypothyroid patients [3, 4]. App. 6% of GD patients demonstrate moderate-to-severe active form. In addition, the sight-threatening GO with dysthyroid optic neuropathy (DON) is observed in fewer than 1% of patients [2]. Full-blown disease is associated with disfiguring features, inflammatory signs and symptoms, visual disturbances, and may lead to permanent visual loss. These characteristic manifestations significantly reduce patients’ health-related quality of life [5,6,7].
Both endogenous and exogenous risk factors may affect the course and severity of GO. Among the non-modifiable factors are gender, age, race, and genetic susceptibility [5, 8, 9]. Although there is a predilection for the female gender, males are more prone to a severe course, especially at the age above 50 years. The environmental factors include thyroid dysfunction, radioactive iodine (RAI) treatment, oxidative stress, and hypercholesterolemia; however, the main modifiable factor is smoking exposure (active and passive) [5, 8, 9]. Tobacco smokers experience more exacerbated ocular symptoms and poorer response to standard immunosuppressive therapy [10, 11]. Elevated thyrotropin-receptor antibodies (TRAbs) titers predispose to similar clinical outcomes [11].
Management of patients with GO requires a multidisciplinary approach based on clinical activity, severity, and disease duration [12]. Also, general measures for all GO patients include eliminating modifiable risk factors. The “wait and watch” strategy and local treatments (artificial tears, gels or ointments and dark glasses) are usually sufficient in mild and active GO. Due to its anti-inflammatory and antioxidant properties, selenium supplementation for 6 months may benefit patients in selenium-deficient areas. In turn, intravenous glucocorticoid pulse therapy (iv GCs) in monotherapy or combined with mycophenolate sodium is considered the first‐line treatment for moderate-to-severe and active GO. The EUGOGO protocol recommends a total cumulative dose of 4.5 g methylprednisolone, given in 12 weekly infusions (0.5 g/week for six weeks, followed by 0.25 g/week for six weeks) [13, 14]. Although effective, this treatment may be accompanied by various adverse events (AEs), such as flushing, hypertension, hyperglycemia, arrhythmias, liver dysfunction, psychosis and infection [10, 15]. However, most reported AEs are associated with single and cumulative doses of methylprednisolone higher than recommended. Moreover, oral therapy is associated with higher steroid-related AEs, including weight gain, hypertension and cushingoid features [16,17,18]. EUGOGO protocol requires twelve hospital admissions, significantly deteriorating patients’ socioeconomic life in our region. Therefore, in 2010 the non-standard protocol was developed, and its safety profile and effectiveness have been investigated in a prospective study (PhD dissertation of Agnieszka Skiba under the supervision of Prof. Jerzy Sowiński).
Our study aimed to assess the safety profile of methylprednisolone administered intravenously for three consecutive days at a dose of 1 g in patients with active, moderate-to-severe or sight-threatening Graves’ orbitopathy.
Materials and methods
Patients
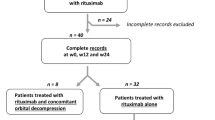
We conducted a retrospective single-centre study evaluating 161 medical records of patients with active, moderate-to-severe and sight-threatening GO treated with high-dose systemic glucocorticoids in the Department of Endocrinology, Metabolic Disorders and Internal Medicine at Poznań University of Medical Sciences in 2014–2021. The treatment protocol consisted of iv methylprednisolone administered for three consecutive days at a dose of 1 g, followed by 600 mg im methylprednisolone in divided doses (cumulative dose, 3.6 g) — Fig. 1. Most patients also received l-ornithine l-aspartate (LOLA) and proton pump inhibitor (PPI) during hospitalization.
Non-standard regimen of systematic steroid therapy of Graves’ orbitopathy in Department of Endocrinology, Metabolic Disorders and Internal Medicine, Poznań University of Medical Sciences, Poznań, Poland. ALT alanine aminotransferase, AST aspartate aminotransferase, im intramuscular, iv intravenous, LOLA l-ornithine l-aspartate, MP methylprednisolone
During qualification for this treatment, each patient underwent a physical examination, followed by an ophthalmological consultation and magnetic resonance imaging of orbits. The clinical activity score (CAS) was used to assess GO severity. Moreover, laboratory tests were performed before and after the iv GCs administration.
Data were obtained from the electronic medical records of the hospital. Clinical data included age, gender, activity and severity of GO, smoking status, duration of the disease, and presented side effects. Also, we collected laboratory results, including Na, K, alanine aminotransferase (ALT), aspartate aminotransferase (AST), thyroid-stimulating hormone (TSH), free triiodothyronine (fT3), free thyroxine (fT4), thyroid-stimulating hormone receptor antibodies (TRAb), thyroid peroxidase antibodies (TPOAb), thyroglobulin antibodies (TgAb), glucose, C-reactive protein (CRP), anti-HCV, HBsAg.
Hyperglycemia was a fasting plasma glucose level of ≥ 100 mg/dL and ≥ 140 mg/dL two hours after meal. We analyzed steroid-induced changes in aminotransferase levels; however, clinically significant aminotransferase increase was defined as ALT or AST elevation by > 3 × the upper limit of normal (ULN).
Ethics statement
We adhered to the ethical standards of the Declaration of Helsinki when we collected, analyzed and reported the data [19]. Due to the retrospective design, the bioethics committee approval is not required.
Statistical analysis
Due to the non-compliance of the continuous variables to the normal distribution (assessed by the Shapiro–Wilk test), the comparisons were made using the Mann–Whitney test or the Wilcoxon test. The Pearson’s Chi-squared test compared distributions of qualitative variables. Univariate and multivariate logistic regression modelling was conducted, describing the odds for the major complications. Also, MPA was used to assess the relationship between major complications and qualitative variables. The significance level was set at α = 0.05 for all analyses. Statistical analysis was performed with Statistica 13.3 (Statsoft, Cracow, Poland).
Results
Characteristics of patients
The analyzed records included patients with moderate-to-severe (n = 110) and sight-threatening (n = 51) Graves’ orbitopathy. Table 1 presents detailed characteristics of recruited patients.
Among the concomitant diseases, the most common were cardiovascular diseases (n = 61), hyperlipidemia (n = 42) and diabetes mellitus (n = 14). Interestingly, diabetes did not affect the presence of steroid-induced hyperglycemia (χ2 = 1.884, df = 1, p = 0.170, Pearson’s Chi-squared test) and elevated TRAb levels (χ2 = 0.370, df = 1, p = 0.543, Pearson’s Chi-squared test).
Comparison of biochemical parameters before and after intravenous steroid therapy
For the whole group, as a result of steroid therapy, significantly increased levels of Na, glucose and ALT, and decreased levels of K and AST were observed (Table 2). Only two patients demonstrated levels of aminotransferases higher than three times the ULN.
Considering LOLA supplementation, pre-treatment AST and ALT levels were not different. However, in the LOLA group, post-treatment AST was significantly lowered (17 vs 21; U = 812, p = 0.033, n1 = 99, n2 = 23, Mann–Whitney test), and ALT was lowered with borderline significance (22.5 vs 28.5; U = 842, p = 0.086, n1 = 100, n2 = 22, Mann–Whitney test) compared to the non-LOLA group.
After the steroid therapy, ALT levels significantly increased in both groups but less in the LOLA group (4.5 vs 8.5; respectively, T = 1280.5, p < 0.001, n = 92, T = 44.5, p = 0.008, n = 22, Wilcoxon test). In contrast, a significant decrease in AST was found in the LOLA group (T = 1429.5, p = 0.004, n = 93, Wilcoxon test), without statistically significant changes in the non-LOLA group (T = 126, p = 0.715, n = 23, Wilcoxon test).
Analyses of steroid therapy-induced side effects
Side effects due to steroid therapy were observed during 116 hospitalizations. The most common were hyperglycemia (n = 95), aminotransferase increase (n = 31), headache (n = 11), facial erythema (n = 11), abdominal pain (n = 5), blood pressure increase (n = 3), delirium (n = 1), resting tremor (n = 2), insomnia (n = 3), peripheral oedema (n = 2).
Distribution comparisons
A higher percentage of hyperglycemia occurred in non-smokers and with normal TRAb levels (at borderline statistical significance). A higher rate of elevated aminotransferases was found in smokers and patients with more than 12 months of disease duration. Also, patients treated for the first time with intravenous steroid therapy and supplemented LOLA appeared to have less often elevated aminotransferase levels (with borderline significance). Detailed results of comparisons are shown in Table 3.
Logistic regression
Logistic regression modelling was performed. Table 4 presents the predictors significant and borderline significant in the univariate analysis for hyperglycemia and elevated aminotransferases, respectively. Lower odds of hyperglycemia were significantly associated with higher CAS values and TRAb levels. Cigarette smoking and disease duration > 1 year indicated significantly more than twofold higher odds of elevated aminotransferases after steroid therapy.
Also, the multivariate regression models were constructed using the stepwise forward technique (Table 5). Again, higher levels of CAS and TRAb protected steroid-induced hyperglycemia (in V-fold cross-validation: training AUC = 0.740 and validation AUC = 0.716). In turn, the GO duration over a year increased the odds of elevated aminotransferases more than 3.5 times, and the male sex decreased by nearly 90% (in V-fold cross-validation: training AUC = 0.842 and validation AUC = 0.799).
Multidimensional correspondence analysis
Based on the MPA, conclusions can be drawn about the relationship of major complications with qualitative variables. Decisions on the number of MPA dimensions were made based on the scree plots. The steroid-induced aminotransferase increase was most strongly associated with smokers and patients who have not supplemented LOLA (Figs. 2 and 3). On the other hand, normal TRAb levels and non-smoking predisposed to hyperglycemia (Fig. 4). Detailed point parameters are reported in Table 6.
Discussion
In our retrospective study, we evaluated the safety profile of a non-standard regimen of 1 g intravenous methylprednisolone (MP) administered for three consecutive days in patients with active, moderate-to-severe or sight-threatening GO. In turn, the recommended treatment for these patients is based on intravenous MP administration on a weekly regimen. However, the cumulative dose of MP should be less than 8 g per cycle, and a single dose of MP should not exceed 0.75 g [13].
The novelty of our study is related to the regimen based on a 3-day intravenous administration of methylprednisolone continued by intramuscular injections, that limits the need for hospitalization and decreases the cumulative dose of steroids. The main reason why administration of methylprednisolone in patients with moderate-to-severe GO over the course of the next 3 days is not recommended is a concern for severe adverse events.
We have demonstrated that 3-day pulses of methylprednisolone do not lead to sudden deaths, liver damage, or thrombosis. Our summary indicated that the applied regimen is relatively safe. A necessary condition is a thorough analysis of risk factors, virological examinations, and metabolic studies. Simultaneously, the regimen limits the need for hospitalization to a few days and allows for the continuation of therapy in outpatient settings. Thyroid orbitopathy is a chronic condition that adversely affects the quality of life, among other things, by worsening socioeconomic conditions [20]. Weekly journeys to a reference center, sometimes located hundreds of km away, are burdensome and exacerbate this situation.
Regardless of the treatment regimen, the clinical use of both short-term and long-term GCs therapy is limited by a wide range of side effects, such as hyperglycemia, insulin resistance, hypertension, arrhythmias, liver dysfunction, psychosis and infections [21, 22]. Also, the frequency and severity of these complications depend on treatment duration, dosage, and route of administration [15]. We reported several mild side effects, such as hyperglycemia, slight aminotransferase increase, headache, and facial erythema. No severe complications, including cardiovascular or hepatic injury, were observed.
GCs therapy is frequently linked with hyperglycemia, glucose intolerance, and diabetes development. Indeed, the effect of GCs on glucose metabolism is a consequence of multiple pathways impairment, such as increased hepatic gluconeogenesis and insulin resistance mainly in the liver and skeletal muscles by interfering with the insulin signaling cascade, as well as through the stimulation of lipolysis and proteolysis [22, 23]. Our study observed that more than half of GO patients presented steroid-induced hyperglycemia. Interestingly, concomitant diabetes did not affect the presence of hyperglycemia. Also, a higher proportion of hyperglycemia appeared in non-smoking patients and patients with normal TRAb levels. Patients with GD have a higher risk of hyperglycemia and diabetes [24]. Also, in the hyperthyroid phase, they have a higher mean amplitude of glycemic excursions [25]. What is more, restoration of thyroid function in those patients leads to deterioration of body composition and visceral fat tissue accumulation [26]. Also, GD and GO are conditions of low-grade inflammation with elevation of circulating adipokines and inflammatory markers [27, 28]. TSH receptors are expressed in the visceral fat tissue in patients with GD and GO and correlate with autoimmunity/inflammation [29]. One may suggest that a higher titer of TRAb may stimulate visceral fat tissue secretory function, leading to adipokines increase and insulin resistance. Those mechanisms could explain the observed association between TRAb titer, CAS and glucose levels.
Cigarette smoking is associated with lower food intake and increased energy expenditure. One of the main causes of increased glucose levels during steroid therapy is an increase in appetite. We could speculate that smokers did not experience that effect that alleviated the influence of steroids on glucose metabolism [30].
Glucose level alterations are observed within hours of GCs exposure and seem dose-dependent [31]. Moreover, Liu et al. found that systematic GCs treatment induces hyperglycemia in 32% and diabetes in 19% of nondiabetic patients [32].
In the literature, no cases of acute liver injury have been reported at cumulative GCs doses up to 8 g [33]. Potential mechanisms of hepatocyte damage include direct dose-dependent toxicity, hypersensitivity reactions and induction of viral or autoimmune hepatitis. In turn, the more commonly observed steroid-induced side effects are asymptomatic elevations of aminotransferases [15, 34,35,36]. Our study found significantly increased ALT and decreased AST after therapy. Moreover, it should be emphasized that only two patients had clinically significant increases in aminotransferases higher than three times the ULN.
However, LOLA supplementation in most patients may be a co-founding factor for these findings. In this group, lower post-treatment levels of both liver enzymes, as well as lower elevations of ALT and a significant decrease in AST, were observed. Previous studies suggest that LOLA administration enhances hepatocytes’ ability to excrete ammonia and improves the functional integrity of the hepatocyte cell membrane. Thus, it prevents hepatocyte damage and the secretion of aminotransferases into the blood [37,38,39].
Although severe GO course is more common for males, we observed significantly reduced odds of elevated aminotransferases in this group. In this case, the less toxic effect of steroids may result from more intense inflammation. In addition, males are characterized by higher body mass, which results in a lower dose of steroids per kg.
Moreover, smoking is associated with a more severe GO, especially treatment-resistant [40]. Xing et al. concluded that even former smoking is an independent risk factor for impaired response to intravenous GCs [41]. Our study showed that smokers with disease duration longer than a year more often demonstrated elevated aminotransferases. This may be related to more extended therapy in patients with a poorer response [42, 43].
Interestingly, Aktaran et al. observed a positive correlation between TRAb levels and inflammatory signs in GO patients smoking more than 20 cigarettes per day during the GCs therapy. It is speculated that smoking alters the structure of thyrotropin receptors, making it more immunogenic and consequently leading to the production of TRAb that reacts with retrobulbar tissues [44, 45].
In general, GCs cause blood pressure increases, but only a few of our patients reported significant increases in blood pressure during the therapy [15, 46]. Previously, Miśkiewicz et al. noticed significant elevated maximal systolic blood pressure and mean nocturnal blood pressure during the last bolus of methylprednisolone in GO therapy [47].
Previous studies describe only long-term complications related to the cumulative dose of GCs, but do not consider the administration of high boluses in GO therapy. The most common side effect include osteoporosis, osteonecrosis, diabetes, metabolic syndrome, cardiovascular diseases, infections and cataract. It is worth noting that the risk of complications such as osteoporosis or diabetes is proven to be dose-dependent. Therefore, it is recommended that the doses of GCs should be as low as possible and administered for as short a period as possible [48,49,50].
Importantly, our Department is a reference centre in GO treatment for two Polish voivodships with more than 4.5 million inhabitants (according to “Area and population in the territorial profile in 2023” by Statistics Poland). Our intravenous 3-day regimen of steroid therapy followed by intramuscular injections, minimizes the frequency of regular hospitalizations with the need to travel to larger medical centres every week. Intramuscular maintenance therapy can occur at the patient’s residence, reducing commuting costs to reference centres. This is important for patients with GO who face physical and mental difficulties daily, which also affects their position in the labor market. Often, they cannot keep their jobs for health reasons, which reduces their social and financial quality of life [20, 51].
Among the limitations of the retrospective study, we are aware of the missing data in medical records. Another limitation was the lack of patient mobility regarding follow-up visits, including ophthalmic examinations, often not carried out in our centre, which is a reference centre for two provinces. Due to the subjective character of some complications, it may be speculated that they had not always been recorded in medical records. The relatively high percentage of patients with the sight-threatening GO may result from the urgent mode of admission in this condition to our Department, and it does not reflect the epidemiological data.
Conclusion
Treatment with high-dose systemic glucocorticoids applied successfully to manage active, moderate-to-severe or sight-threatening Graves’ orbitopathy, leads to mild side effects, mainly manifested by hyperglycemia and elevated liver enzymes. However, the increased aminotransferase values do not indicate steroid-induced severe liver injury. Thus, the treatment should be carried out in specialized centres, and laboratory parameters need to be monitored during the treatment.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AEs:
-
Adverse events
- ALT:
-
Alanine aminotransferase
- ALT-1:
-
Alanine aminotransferase before steroid therapy
- AST:
-
Aspartate aminotransferase
- AST-1:
-
Aspartate aminotransferase before steroid therapy
- BMI:
-
Body mass index
- CAS:
-
Clinical activity score
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- DON:
-
Dysthyroid optic neuropathy
- fT3:
-
Free triiodothyronine
- fT4:
-
Free thyroxine
- GCs:
-
Glucocorticoids
- GD:
-
Graves’ disease
- GO:
-
Graves’ orbitopathy
- im :
-
Intramuscular
- iv :
-
Intravenous
- iv GCs:
-
Intravenous glucocorticoid pulse therapy
- LOLA:
-
L-ornithine l-aspartate
- MP:
-
Methylprednisolone
- MPA:
-
Multidimensional correspondence analysis
- OR:
-
Odds ratio
- PPI:
-
Proton pump inhibitor
- RAI:
-
Radioactive iodine
- SE:
-
Standard error
- TAO:
-
Thyroid-associated ophthalmopathy
- TED:
-
Thyroid eye disease
- TgAb:
-
Thyroglobulin antibodies
- TPOAb:
-
Thyroid peroxidase antibodies
- TRAb:
-
Thyroid-stimulating hormone receptor antibodies
- TRAbs:
-
Thyrotropin-receptor antibodies
- TSH:
-
Thyroid-stimulating hormone
- ULN:
-
Upper limit of normal
References
Salvi M, Campi I. Medical treatment of Graves’ orbitopathy. Horm Metab Res. 2015;47:779–88.
Melcescu E, Horton WB, Kim D, Vijayakumar V, Corbett JJ, Crowder KW, et al. Graves orbitopathy: update on diagnosis and therapy. South Med J. 2014;107:34–43.
Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38.
Li X, Li S, Fan W, Rokohl AC, Ju S, Ju X, et al. Recent advances in graves ophthalmopathy medical therapy: a comprehensive literature review. Int Ophthalmol. 2023;43:1437–49.
Bartalena L, Tanda ML. Current concepts regarding Graves’ orbitopathy. J Intern Med. 2022;292:692–716.
Gontarz-Nowak K, Szychlińska M, Matuszewski W, Stefanowicz-Rutkowska M, Bandurska-Stankiewicz E. Current knowledge on Graves’ orbitopathy. J Clin Med. 2020;10:16.
Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375:1552–65.
Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of graves’ Orbitopathy. Front Endocrinol. 2020;11: 615993.
Zawadzka-Starczewska K, Stasiak B, Wojciechowska-Durczyńska K, Lewiński A, Stasiak M. Novel insight into non-genetic risk factors of Graves’ Orbitopathy. Int J Environ Res Public Health. 2022;19:16941.
Genere N, Stan MN. Current and emerging treatment strategies for Graves’ orbitopathy. Drugs. 2019;79:109–24.
Roos JCP, Paulpandian V, Murthy R. Serial TSH-receptor antibody levels to guide the management of thyroid eye disease: the impact of smoking, immunosuppression, radio-iodine, and thyroidectomy. Eye. 2019;33:212–7.
Ruchała M, Sawicka-Gutaj N. Advances in the pharmacological treatment of Graves’ orbitopathy. Expert Rev Clin Pharmacol. 2016;9:981–9.
Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185:43–67.
Nowak M, Marek B, Kos-Kudła B, Siemińska L, Londzin-Olesik M, Głogowska-Szeląg J, et al. Optimization of the treatment of moderate to severe and active thyroid orbitopathy considering the recommendations of the European Group on Graves’ Orbitopathy (EUGOGO). Endokrynol Pol. 2022;73:756–77.
Längericht J, Krämer I, Kahaly GJ. Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application. Ther Adv Endocrinol Metab. 2020;11:2042018820958335.
Stiebel-Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94:2708–16.
Riedl M, Kolbe E, Kampmann E, Krämer I, Kahaly GJ. Prospectively recorded and MedDRA-coded safety data of intravenous methylprednisolone therapy in Graves’ orbitopathy. J Endocrinol Invest. 2015;38:177–82.
Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40.
Sawicka-Gutaj N, Gruszczyński D, Guzik P, Mostowska A, Walkowiak J. Publication ethics of human studies in the light of the Declaration of Helsinki – a mini-review. J Med Sci. 2022;91:700.
Ponto KA, Merkesdal S, Hommel G, Pitz S, Pfeiffer N, Kahaly GJ. Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98:145–52.
Vandewalle J, Luypaert A, Bosscher KD, Libert C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol Metab. 2018;29:42–54.
Beaupere C, Liboz A, Fève B, Blondeau B, Guillemain G. Molecular mechanisms of glucocorticoid-induced insulin resistance. Int J Mol Sci. 2021;22:623.
Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: prevalence, early detection and therapeutic recommendations: a narrative review. World J Diabetes. 2015;6:1073–81.
Song E, Koo MJ, Noh E, Hwang SY, Park MJ, Kim JA, et al. Risk of diabetes in patients with long-standing Graves’ disease: a longitudinal study. Endocrinol Metab Seoul Korea. 2021;36:1277–86.
Gao G, Li FF, Hu Y, Yan RN, Liu BL, Liu XM, et al. Glycemic variation in uncontrolled Graves’ disease patients with normal glucose metabolism: assessment by continuous glucose monitoring. Endocrine. 2019;64:265–70.
Sawicka-Gutaj N, Zybek-Kocik A, Kloska M, Ziółkowska P, Czarnywojtek A, Sowiński J, et al. Effect of restoration of euthyroidism on visfatin concentrations and body composition in women. Endocr Connect. 2021;10:462–70.
Sawicka-Gutaj N, Zybek-Kocik A, Kloska M, Czarnywojtek A, Sowiński J, Budny B, et al. Determinants of Visfatin/NAMPT serum concentration and its leukocyte expression in hyperthyroidism. Horm Metab Res Horm Stoffwechselforschung Horm Metab. 2018;50:653–60.
Falkowski B, Szczepanek-Parulska E, Sawicka-Gutaj N, Krygier A, Ruchala M. Evaluation of IL-29 in euthyroid patients with Graves’ orbitopathy: a preliminary study. Mediators Inflamm. 2020;2020:4748612.
Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M. Adipose thyrotrophin receptor expression is elevated in Graves’ and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. J Mol Endocrinol. 2003;30:369–80.
Schwartz A, Bellissimo N. Nicotine and energy balance: a review examining the effect of nicotine on hormonal appetite regulation and energy expenditure. Appetite. 2021;164: 105260.
Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9:30.
Liu X, Zhu X, Miao Q, Ye H, Zhang Z, Li Y. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65:324–32.
Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt-Rasmussen U, Orgiazzi J, et al. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol. 2012;166:247–53.
Miśkiewicz P, Kryczka A, Ambroziak U, Rutkowska B, Główczyńska R, Opolski G, et al. Is high dose intravenous methylprednisolone pulse therapy in patients with Graves’ orbitopathy safe? Endokrynol Pol. 2014;65:402–13.
Zang S, Ponto KA, Kahaly GJ. Clinical review: Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96:320–32.
Wichary H, Gasińska T. Methylprednisolone and hepatotoxicity in Graves’ ophthalmopathy. Thyroid. 2012;22:64–9.
Najmi AK, Pillai KK, Pal SN, Akhtar M, Aqil M, Sharma M. Effect of l-ornithine l-aspartate against thioacetamide-induced hepatic damage in rats. Indian J Pharmacol. 2010;42:384–7.
Butterworth RF, Canbay A. Hepatoprotection by L-ornithine L-aspartate in non-alcoholic fatty liver disease. Dig Dis Basel Switz. 2019;37:63–8.
Kircheis G, Lüth S. Pharmacokinetic and pharmacodynamic properties of L-ornithine L-aspartate (LOLA) in hepatic encephalopathy. Drugs. 2019;79:23–9.
Hoppe E, Lee ACH, Hoppe D, Kahaly GJ. Predictive factors for changes in quality of life after steroid treatment for active moderate-to-severe Graves’ orbitopathy: a prospective trial. Eur Thyroid J. 2021;9:313–20.
Xing L, Ye L, Zhu W, Shen L, Huang F, Jiao Q, et al. Smoking was associated with poor response to intravenous steroids therapy in Graves’ ophthalmopathy. Br J Ophthalmol. 2015;99:1686–91.
Kemchoknatee P, Tangon D, Srisombut T. A single-center analysis of visual outcomes and associated factors after intravenous methylprednisolone treatment for dysthyroid optic neuropathy. BMC Ophthalmol. 2023;23:32.
Miśkiewicz P, Rutkowska B, Jabłońska A, Krzeski A, Trautsolt-Jeziorska K, Kęcik D, et al. Complete recovery of visual acuity as the main goal of treatment in patients with dysthyroid optic neuropathy. Endokrynol Pol. 2016;67:166–73.
Aktaran S, Akarsu E, Erbağci I, Araz M, Okumuş S, Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy. Int J Clin Pract. 2007;61:45–51.
Utiger RD. Effects of smoking on thyroid function. Eur J Endocrinol. 1998;138:368–9.
Imaizumi T, Nakatochi M, Fujita Y, Yamamoto R, Watanabe K, Maekawa M, et al. Glucocorticoid treatment is associated with ICU-acquired hypernatremia: a nested case-control study. Clin Exp Nephrol. 2021;25:131–9.
Miskiewicz P, Milczarek-Banach J, Bednarczuk T, Opolski G, Glowczynska R. Blood pressure profile and N-terminal-proBNP dynamics in response to intravenous methylprednisolone pulse therapy of severe Graves’ orbitopathy. Int J Mol Sci. 2018;19:2918.
Li JX, Cummins CL. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat Rev Endocrinol. 2022;18:540–57.
Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020;16:437–47.
Seguro LPC, Rosario C, Shoenfeld Y. Long-term complications of past glucocorticoid use. Autoimmun Rev. 2013;12:629–32.
Leso V, Vetrani I, De Cicco L, Cardelia A, Fontana L, Buonocore G, et al. The impact of thyroid diseases on the working life of patients: a systematic review. Int J Environ Res Public Health. 2020;17:4295.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
NS-G: conceived the concept of the study and contributed to the design of the research. DG, NZ and MP were involved in data collection. KN: analyzed the data. NS-G, DG, NZ, and KN: drafted the article. AS, JS, MR: revised the manuscript for final submission. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sawicka-Gutaj, N., Gruszczyński, D., Zawalna, N. et al. Safety of non-standard regimen of systemic steroid therapy in patients with Graves’ orbitopathy: a single-centre experience. Pharmacol. Rep 76, 185–194 (2024). https://doi.org/10.1007/s43440-023-00567-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00567-0