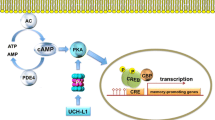
Abstract
Neurodegeneration is a condition of the central nervous system (CNS) characterized by loss of neural structures and function. The most common neurodegenerative disorders (NDDs) include Alzheimer's disease (AD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), multiple sclerosis (MS), motor neuron disorders, psychological disorders, dementia with vascular dementia (VaD), Lewy body dementia (DLB), epilepsy, cerebral ischemia, mental illness, and behavioral disorders. CREB (cAMP-response element-binding protein) represent a nuclear protein that regulates gene transcriptional activity. The primary focus of the review pertains to the exploration of CREB expression and activation within the context of neurodegenerative diseases, specifically in relation to the phosphorylation and dephosphorylation events that occur within the CREB signaling pathway under normal physiological conditions. The findings mentioned have contributed to the elucidation of the regulatory mechanisms governing CREB activity. Additionally, they have provided valuable insights into the potential mediation of diverse biological processes, such as memory consolidation and neuroprotective effects, by various related studies. The promotion of synaptic plasticity and neurodevelopment in the central nervous system through the targeting of CREB proteins has the potential to contribute to the prevention or delay of the onset of neurodegenerative disorders. Multiple drugs have been found to initiate downstream signaling pathways, leading to neuroprotective advantages in both animal model studies and clinical trials. The clinical importance of the cAMP-response element-binding protein (CREB) is examined in this article, encompassing its utility as both a predictive/prognostic marker and a target for therapeutic interventions.
Graphical abstract





Similar content being viewed by others
Availability of data and material
Not applicable.
Abbreviations
- ATF1:
-
Activation transcription factor 1
- BDNF:
-
Brain-derived neurotrophic factor
- bZIP:
-
Basic leucine zipper
- CaMKIV:
-
Ca2+/calmodulin-dependent protein kinase IV
- CaN:
-
Calcineurin
- CREB:
-
CAMP-responsive element-binding protein
- CREM:
-
CAMP-responsive element modulator
- GPCRs:
-
G-protein-coupled receptors
- JNK:
-
C-Jun N-terminal kinase pathway
- KID:
-
Kinase inducible domain
- MAPK:
-
Mitogen-activated protein kinase
- NDDs:
-
Neurodegenerative disorders
- NF-κB:
-
NF-kappa B, “nuclear factor kappa-light-chain-enhancer of activated B cells”
- NGF:
-
Nerve growth factor
- NMDARs:
-
N-Methyl-D-aspartate receptors
- PACAP:
-
Pituitary adenylate cyclase-activating polypeptide
- PKA:
-
Protein kinase A
- PP-1:
-
Protein phosphatase-1
- RTKs:
-
Receptor tyrosine kinases
References
Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010;14(3):457–87. https://doi.org/10.1111/j.1582-4934.2010.01010.x.
Khan H, Garg N, Singh TG, Kaur A, Thapa K. Calpain inhibitors as potential therapeutic modulators in neurodegenerative diseases. Neurochem Res. 2022;4:1–25. https://doi.org/10.1007/s11064-021-03521-9.
Khan H, Tiwari P, Kaur A, Singh TG. Sirtuin acetylation and deacetylation: a complex paradigm in neurodegenerative disease. Mol Neurobiol. 2021;58(8):3903–17. https://doi.org/10.1007/s12035-021-02387-w.
Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2017;9(7):a028035. https://doi.org/10.1101/cshperspect.a028035.
Behl T, Kaur G, Sehgal A, Bhardwaj S, Singh S, Buhas C, et al. Multifaceted role of matrix metalloproteinases in neurodegenerative diseases: Pathophysiological and therapeutic perspectives. International Int J Mol Sci. 2021;22(3):1413. https://doi.org/10.3390/ijms22031413.
Bhattacharya T, Soares GA, Chopra H, Rahman MM, Hasan Z, Swain SS, et al. Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. Materials. 2022;15(3):804. https://doi.org/10.3390/ma15030804.
Camandola S, Mattson MP. NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11(2):123–32. https://doi.org/10.1517/14728222.11.2.123.
Schumacher MA, Goodman RH, Brennan RG. The structure of a CREB bZIP· somatostatin CRE complex reveals the basis for selective dimerization and divalent cation-enhanced DNA binding. J Biol Chem. 2000;275(45):35242–7. https://doi.org/10.1074/jbc.M007293200.
Yin Y, Gao D, Wang Y, Wang ZH, Wang X, Ye J, et al. Tau accumulation induces synaptic impairment and memory deficit by calcineurin-mediated inactivation of nuclear CaMKIV/CREB signaling. Proc Natl Acad Sci. 2016;113(26):E3773–81. https://doi.org/10.1073/pnas.1604519113.
Grewal AK, Singh TG, Sharma D, Sharma V, Singh M, Rahman MH, et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed Pharmacother. 2021;140:111729. https://doi.org/10.1016/j.biopha.2021.111729.
Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Villalba AM, et al. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31(1):47–54. https://doi.org/10.1038/ng882.
Lu KT, Chiou RY, Chen LG, Chen MH, Tseng WT, Hsieh HT, et al. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54(8):3126–31. https://doi.org/10.1021/jf053011q.
Barco A, Pittenger C, Kandel ER. CREB, memory enhancement and the treatment of memory disorders: promises, pitfalls and prospects. Expert Opin Ther Targets. 2003;7(1):101–14. https://doi.org/10.1517/14728222.7.1.101.
Gong Y, Chen J, Jin Y, Wang C, Zheng M, He L. GW9508 ameliorates cognitive impairment via the cAMP-CREB and JNK pathways in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuropharmacology. 2020;164:107899. https://doi.org/10.1016/j.neuropharm.2019.107899.
Behl T, Kaur D, Sehgal A, Singh S, Sharma N, Zengin G, et al. Role of monoamine oxidase activity in Alzheimer’s disease: an insight into the therapeutic potential of inhibitors. Molecules. 2021;26(12):3724. https://doi.org/10.3390/molecules26123724.
Rani L, Kaushal J, Srivastav AL, Mahajan P. A critical review on recent developments in MOF adsorbents for the elimination of toxic heavy metals from aqueous solutions. Environ Sci Pollut Res. 2020;27:44771–96. https://doi.org/10.1007/s11356-020-10738-8.
Du Q, Zhu X, Si J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp Biol Med. 2020;245(1):1. https://doi.org/10.1177/1535370219894558.
Wang H, Xu J, Lazarovici P, Quirion R, Zheng W. cAMP response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front Mol Neurosci. 2018;30(11):255. https://doi.org/10.3389/fnmol.2018.00255.
Sharma A, Khan H, Singh TG, Grewal AK, Najda A, Kawecka-Radomska M, et al. Pharmacological modulation of ubiquitin-proteasome pathways in oncogenic signaling. Int J Mol Sci. 2021;22(21):11971. https://doi.org/10.3390/ijms222111971.
Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116(1):1–9. https://doi.org/10.1111/j.1471-4159.2010.07080.x.
Singh S, Singh TG. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: an mechanistic approach. Curr Neuropharmacol. 2020;18(10):918–35. https://doi.org/10.2174/1570159X18666200207120949.
Nagu P, Parashar A, Behl T, Mehta V. CNS implications of COVID-19: a comprehensive review. Rev Neurosci. 2021;32(2):219–34. https://doi.org/10.1515/revneuro-2020-0070.
Gonzalez J, Jurado-Coronel JC, Avila MF, Sabogal A, Capani F, Barreto GE. NMDARs in neurological diseases: a potential therapeutic target. Int J Neurosci. 2015;125(5):315–27. https://doi.org/10.3109/00207454.2014.940941.
Zhao X, Li S, Gaur U, Zheng W. Artemisinin improved neuronal functions in Alzheimer’s disease animal model 3xtg mice and neuronal cells via stimulating the ERK/CREB signaling pathway. Aging Dis. 2020;11(4):801. https://doi.org/10.14336/AD.2019.0813.
Feng H, Wang C, He W, Wu X, Li S, Zeng Z, et al. Roflumilast ameliorates cognitive impairment in APP/PS1 mice via cAMP/CREB/BDNF signaling and anti-neuroinflammatory effects. Metab Brain Dis. 2019;34(2):583–91. https://doi.org/10.1007/s11011-018-0374-4.
Wang CY, Wang ZY, Xie JW, Wang T, Wang X, Xu Y, et al. Dl-3-n-butylphthalide-induced upregulation of antioxidant defense is involved in the enhancement of cross talk between CREB and Nrf2 in an Alzheimer’s disease mouse model. Neurobiol Aging. 2016;1(38):32–46. https://doi.org/10.1016/j.neurobiolaging.2015.10.024.
Arora A, Behl T, Sehgal A, Singh S, Sharma N, Bhatia S, et al. Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 2021;15(273):119311. https://doi.org/10.1016/j.lfs.2021.119311.
Rosa E, Fahnestock M. CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol Aging. 2015;36(8):2406–13. https://doi.org/10.1016/j.neurobiolaging.2015.04.014.
Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+-and stimulus duration–dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–14. https://doi.org/10.1016/s0092-8674(00)81816-4.
Saklani P, Khan H, Gupta S, Kaur A, Singh TG. Neuropeptides: potential neuroprotective agents in ischemic injury. Life Sci. 2022;288:120186. https://doi.org/10.1016/j.lfs.2021.120186.
Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45. https://doi.org/10.1016/j.tins.2005.06.005.
Gupta A, Khan H, Kaur A, Singh TG. Novel targets explored in the treatment of alcohol withdrawal syndrome. CNS Neurol Disord Drug Targets. 2021;20(2):158–73. https://doi.org/10.2174/1871527319999201118155721.
Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, et al. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002;22(22):9868–76. https://doi.org/10.1523/JNEUROSCI.22-22-09868.2002.
Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286(5448):2358–61. https://doi.org/10.1126/science.286.5448.2358.
Kievit P, Maurer RA. The pituitary-specific transcription factor, Pit-1, can direct changes in the chromatin structure of the prolactin promoter. Mol Endocrinol. 2005;19(1):138–47. https://doi.org/10.1210/me.2004-0016.
Kowalczyk A, Filipkowski RK, Rylski M, Wilczynski GM, Konopacki FA, Jaworski J, et al. The critical role of cyclin D2 in adult neurogenesis. J Cell Biol. 2004;167(2):209–13. https://doi.org/10.1083/jcb.200404181.
Amidfar M, de Oliveira J, Kucharska E, Budni J, Kim YK. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020;257:118020. https://doi.org/10.1016/j.lfs.2020.118020.
Sharma B, Sharma A. Future prospect of nanotechnology in development of anti-ageing formulations. Int J Pharm Pharm Sci. 2012;4(3):57–66.
Garg N, Singh TG, Khan H, Arora S, Kaur A, Mannan A. Mechanistic interventions of selected ocimum species in management of diabetes, obesity and liver disorders: transformative developments from preclinical to clinical approaches. Biointerface Res Appl Chem. 2021;12(1):1304–23. https://doi.org/10.33263/BRIAC121.13041323.
Khan H, Sharma K, Kumar A, Kaur A, Singh TG. Therapeutic implications of cyclooxygenase (COX) inhibitors in ischemic injury. Inflamm Res. 2022;17:1–6. https://doi.org/10.1007/s00011-022-01546-6.
Wang DD, Li J, Yu LP, Wu MN, Sun LN, Qi JS. Desipramine improves depression-like behavior and working memory by up-regulating p-CREB in Alzheimer’s disease associated miceJ. Integr Neurosci. 2016;15(02):247–60. https://doi.org/10.1142/S021963521650014X.
Bae HJ, Sowndhararajan K, Park HB, Kim SY, Kim S, Kim DH, et al. Danshensu attenuates scopolamine and amyloid-β-induced cognitive impairments through the activation of PKA-CREB signaling in mice. Neurochem Int. 2019;131:104537. https://doi.org/10.1016/j.neuint.2019.104537.
Khan H, Singh A, Thapa K, Garg N, Grewal AK, Singh TG. Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res. 2021;1761:147399. https://doi.org/10.1016/j.brainres.2021.147399.
Hosseini L, Mahmoudi J, Pashazadeh F, Salehi-Pourmehr H, Sadigh-Eteghad S. Protective effects of nicotinamide adenine dinucleotide and related precursors in Alzheimer’s disease: a systematic review of preclinical studies. J Mol Neurosci. 2021;71(7):1425–35. https://doi.org/10.1007/s12031-021-01842-6.
Shukla PK, Sandhu JK, Ahirwar A, Ghai D, Maheshwary P, Shukla PK. Multiobjective genetic algorithm and convolutional neural network based COVID-19 identification in chest X-ray images. Math Probl Eng. 2021;20(2021):1–9. https://doi.org/10.1155/2021/7804540.
DeMaagd G, Philip A. Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharm Ther. 2015;40(8):504.
Labadorf A, Choi SH, Myers RH. Evidence for a pan-neurodegenerative disease response in Huntington’s and Parkinson’s disease expression profiles. Front Mol Neurosci. 2018;11(10):430. https://doi.org/10.3389/fnmol.2017.00430.
Wu X, Liang Y, Jing X, Lin D, Chen Y, Zhou T, et al. Rifampicin prevents SH-SY5Y cells from rotenone-induced apoptosis via the PI3K/Akt/GSK-3β/CREB signaling pathway. Neurochem Res. 2018;43(4):886–93. https://doi.org/10.1007/s11064-018-2494-y.
Zhong J, Dong W, Qin Y, Xie J, Xiao J, Xu J, et al. Roflupram exerts neuroprotection via activation of CREB/PGC-1α signalling in experimental models of Parkinson’s disease. Br J Pharmacol. 2020;177(10):2333–50. https://doi.org/10.1111/bph.14983.
Sun C, Wang Y, Mo M, Song C, Wang X, Chen S, et al. Minocycline protects against rotenone-induced neurotoxicity correlating with upregulation of Nurr1 in a Parkinson’s disease rat model. Biomed Res Int. 2019;5:2019. https://doi.org/10.1155/2019/6843265.
Zheng M, Liu C, Fan Y, Shi D, Jian W. Total glucosides of paeony (TGP) extracted from Radix Paeoniae Alba exerts neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the cAMP/PKA/CREB signaling pathway. J Ethnopharmacol. 2019;5(245):112182. https://doi.org/10.1016/j.jep.2019.112182.
Khan H, Kashyap A, Kaur A, Singh TG. Pharmacological postconditioning: a molecular aspect in ischemic injury. J Pharm Pharmacol. 2020;72(11):1513–27. https://doi.org/10.1111/jphp.13336.
Rihal V, Khan H, Kaur A, Singh TG. Vitamin D as therapeutic modulator in cerebrovascular diseases: a mechanistic perspectives. Crit Rev Food Sci Nutr. 2022;5:1–23. https://doi.org/10.1080/10408398.2022.2050349.
Khan H, Grewal AK, Singh TG. Pharmacological postconditioning by protocatechuic acid attenuates brain injury in ischemia-reperfusion (I/R) mice model: implications of nuclear factor erythroid-2-related factor pathway. Neuroscience. 2022. https://doi.org/10.1016/j.neuroscience.2022.03.016.
Hu S, Cao Q, Xu P, Ji W, Wang G, Zhang Y. Rolipram stimulates angiogenesis and attenuates neuronal apoptosis through the cAMP/cAMP-responsive element binding protein pathway following ischemic stroke in rats. Exp Ther Med. 2016;11(3):1005–10. https://doi.org/10.3892/etm.2015.2958.
Li S, Peng T, Zhao X, Silva M, Liu L, Zhou W, et al. Artemether confers neuroprotection on cerebral ischemic injury through stimulation of the Erk1/2-P90rsk-CREB signaling pathway. Redox Biol. 2021;46:102069. https://doi.org/10.1016/j.redox.2021.102069.
Wang H, Zhang Y, Li H, Zeng W, Qiao M. Shuyu capsules relieve liver-qi depression by regulating ERK-CREB-BDNF signal pathway in central nervous system of rat. Exp Ther Med. 2017;14(5):4831–8. https://doi.org/10.3892/etm.2017.5125.
Bu F, Min JW, Munshi Y, Lai YJ, Qi L, Urayama A, et al. Activation of endothelial ras-related C3 botulinum toxin substrate 1 (Rac1) improves post-stroke recovery and angiogenesis via activating Pak1 in mice. Exp Neurol. 2019;322:113059. https://doi.org/10.1016/j.expneurol.2019.113059.
Takagi T, Hara H. Protective effects of cilostazol against hemorrhagic stroke: current and future perspectives. J Pharmacol Sci. 2016;131(3):155–61. https://doi.org/10.1016/j.jphs.2016.04.023.
Barfejani AH, Jafarvand M, Seyedsaadat SM, Rasekhi RT. Donepezil in the treatment of ischemic stroke: review and future perspective. Life Sci. 2020;263:118575. https://doi.org/10.1016/j.lfs.2020.118575.
Etgen AM, Jover-Mengual T, Zukin RS. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: translational implications. Front Neuroendocrinol. 2011;32(3):336–52. https://doi.org/10.1016/j.yfrne.2010.12.005.
Chaturvedi RK, Hennessey T, Johri A, Tiwari SK, Mishra D, Agarwal S, et al. Transducer of regulated CREB-binding proteins (TORCs) transcription and function is impaired in Huntington’s disease. Hum Mol Genet. 2012;21(15):3474–88. https://doi.org/10.1093/hmg/dds178.
Choi YS, Lee B, Cho HY, Reyes IB, Pu XA, Saido TC, et al. CREB is a key regulator of striatal vulnerability in chemical and genetic models of Huntington’s disease. Neurobiol Dis. 2009;36(2):259–68. https://doi.org/10.1016/j.nbd.2009.07.014.
Lee M, Ban JJ, Chung JY, Im W, Kim M. Amelioration of Huntington’s disease phenotypes by Beta-Lapachone is associated with increases in Sirt1 expression, CREB phosphorylation and PGC-1α deacetylation. PLoS ONE. 2018;13(5):e0195968. https://doi.org/10.1371/journal.pone.0195968.
Paldino E, Balducci C, La Vitola P, Artioli L, D’Angelo V, Giampà C, et al. Neuroprotective effects of doxycycline in the R6/2 mouse model of Huntington’s disease. Mol Neurobiol. 2020;57(4):1889–903. https://doi.org/10.1007/s12035-019-01847-8.
Sayed NH, Fathy N, Kortam MA, Rabie MA, Mohamed AF, Kamel AS. Vildagliptin attenuates Huntington’s disease through activation of GLP-1 receptor/PI3K/Akt/BDNF pathway in 3-nitropropionic acid rat model. Neurotherapeutics. 2020;17(1):252–68. https://doi.org/10.1007/s13311-019-00805-5.
Mehan S, Parveen S, Kalra S. Adenyl cyclase activator forskolin protects against Huntington’s disease-like neurodegenerative disorders. Neural Regen Res. 2017;12(2):290. https://doi.org/10.4103/1673-5374.200812.
Paldino E, D’Angelo V, Laurenti D, Angeloni C, Sancesario G, Fusco FR. Modulation of inflammasome and pyroptosis by olaparib, a PARP-1 inhibitor, in the R6/2 mouse model of Huntington’s disease. Cells. 2020;9(10):2286. https://doi.org/10.3390/cells9102286.
Chen B, An J, Guo YS, Tang J, Zhao JJ, Zhang R, et al. Tetramethylpyrazine induces the release of BDNF from BM-MSCs through activation of the PI3K/AKT/CREB pathway. Cell Biol Int. 2021;45(12):2429–42. https://doi.org/10.1002/cbin.11687.
Ding G, Zhao J, Jiang D. Allicin inhibits oxidative stress-induced mitochondrial dysfunction and apoptosis by promoting PI3K/AKT and CREB/ERK signaling in osteoblast cells. Exp Ther Med. 2016;11(6):2553–60. https://doi.org/10.3892/etm.2016.3179.
Khan H, Gupta A, Singh TG, Kaur A. Mechanistic insight on the role of leukotriene receptors in ischemic–reperfusion injury. Pharmacol Rep. 2021;73(5):1240–54. https://doi.org/10.1007/s43440-021-00258-8.
Danduga RC, Dondapati SR, Kola PK, Grace L, Tadigiri RV, Kanakaraju VK. Neuroprotective activity of tetramethylpyrazine against 3-nitropropionic acid induced Huntington’s disease-like symptoms in rats. Biomed Pharmacother. 2018;105:1254–68. https://doi.org/10.1016/j.biopha.2018.06.079.
Kreilaus F, Spiro AS, Hannan AJ, Garner B, Jenner AM. Therapeutic effects of anthocyanins and environmental enrichment in R6/1 Huntington’s disease mice. J Huntingt Dis. 2016;5(3):285–96. https://doi.org/10.3233/JHD-160204.
Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377(2):162–72. https://doi.org/10.1056/NEJMra1603471.
Ding ML, Ma H, Man YG, Lv HY. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can J Physiol Pharmacol. 2017;95(12):1396–405. https://doi.org/10.1139/cjpp-2016-0333.
Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. The Lancet. 2015;385(9971):884–98. https://doi.org/10.1016/S0140-6736(14)60456-6.
Wang G, Zhu Z, Xu D, Sun L. Advances in understanding CREB signaling-mediated regulation of the pathogenesis and progression of epilepsy. Clin Neurol Neurosurg. 2020;196:106018. https://doi.org/10.1016/j.clineuro.2020.106018.
Zhen JL, Chang YN, Qu ZZ, Fu T, Liu JQ, Wang WP. Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav. 2016;1(57):177–84. https://doi.org/10.1016/j.yebeh.2016.02.001.
Ping X, Qin SK, Liu SN, Lu Y, Zhao YN, Cao YF, et al. Effects of Huazhuo Jiedu Shugan Decoction on cognitive and emotional disorders in a rat model of epilepsy: possible involvement of AC-cAMP-CREB signaling and NPY expression. Evid Based Complement Alternat Med. 2019;13:2019. https://doi.org/10.1155/2019/4352879.
Yu X, Guan Q, Wang Y, Shen H, Zhai L, Lu X, et al. Anticonvulsant and anti-apoptosis effects of salvianolic acid B on pentylenetetrazole-kindled rats via AKT/CREB/BDNF signaling. Epilepsy Res. 2019;1(154):90–6. https://doi.org/10.1016/j.eplepsyres.2019.05.007.
Sharma P, Kumari S, Sharma J, Purohit R, Singh D. Hesperidin interacts with CREB-BDNF signaling pathway to suppress pentylenetetrazole-induced convulsions in zebrafish. Front Pharmacol. 2021;11(11):2178. https://doi.org/10.3389/fphar.2020.607797.
Tavassoli M, Ardjmand A. Pentylenetetrazol and morphine interaction in a state-dependent memory model: role of CREB signaling. Basic Clin Neurosci. 2020;11(4):557. https://doi.org/10.32598/bcn.11.4.1482.1.
Ullah I, Badshah H, Naseer MI, Lee HY, Kim MO. Thymoquinone and vitamin C attenuates pentylenetetrazole-induced seizures via activation of GABAB1 receptor in adult rats cortex and hippocampus. Neuromol Med. 2015;17(1):35–46. https://doi.org/10.1007/s12017-014-8337-3.
Lu Y, Wang X, Feng J, Xie T, Si P, Wang W. Neuroprotective effect of astaxanthin on newborn rats exposed to prenatal maternal seizures. Brain Res Bull. 2019;1(148):63–9. https://doi.org/10.1016/j.brainresbull.2019.03.009.
Sawamoto A, Okuyama S, Nakajima M, Furukawa Y. Citrus flavonoid 3, 5, 6, 7, 8, 3′, 4′-heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol Rep. 2019;71(4):653–8. https://doi.org/10.1016/j.pharep.2019.03.006.
Namgyal D, Ali S, Mehta R, Sarwat M. The neuroprotective effect of curcumin against Cd-induced neurotoxicity and hippocampal neurogenesis promotion through CREB-BDNF signaling pathway. Toxicology. 2020;442:152542. https://doi.org/10.1016/j.tox.2020.152542.
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India for providing the necessary facilities to carry out the research work.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization: TGS. Wrote the manuscript: NK, HK. Editing of the manuscript: HK, AK, TGS. Critically reviewed the article: TGS. Supervision: TGS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Ethical standards
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khakha, N., Khan, H., Kaur, A. et al. Therapeutic implications of phosphorylation- and dephosphorylation-dependent factors of cAMP-response element-binding protein (CREB) in neurodegeneration. Pharmacol. Rep 75, 1152–1165 (2023). https://doi.org/10.1007/s43440-023-00526-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00526-9