Abstract
Although Warburg's discovery of intensive glucose uptake by tumors, followed by lactate fermentation in oxygen presence of oxygen was made a century ago, it is still an area of intense research and development of new hypotheses that, layer by layer, unravel the complexities of neoplastic transformation. This seemingly simple metabolic reprogramming of cancer cells reveals an intriguing, multi-faceted nature that may link various phenomena including cell signaling, cell proliferation, ROS generation, energy supply, macromolecules synthesis/biosynthetic precursor supply, immunosuppression, or cooperation of cancerous cells with cancer-associated fibroblasts (CAFs), known as reversed Warburg effect. According to the current perception of the causes and consequences of the Warburg effect, PI3K/Akt/mTOR are the main signaling pathways that, in concert with the transcription factors HIF-1, p53, and c-Myc, modulate the activity/expression of key regulatory enzymes, including PKM2, and PDK1 to tune in the most optimal metabolic setting for the cancer cell. This in turn secures adequate levels of biosynthetic precursors, NADPH, NAD+, and rapid ATP production to meet the increased demands of intensively proliferating tumor cells. The end-product of “aerobic glycolysis”, lactate, an oncometabolite, may provide fuel to neighboring cancer cells, and facilitate metastasis and immunosuppression together enabling cancer progression. The importance and possible applicability of the presented issue are best illustrated by numerous trials with various agents targeting the Warburg effect, constituting a promising strategy in future anti-cancer regimens. In this review, we present the key aspects of this multifactorial phenomenon, depicting the mechanisms and benefits behind the Warburg effect, and also pointing to selected aspects in the field of anticancer therapy.
Similar content being viewed by others
Introduction
Cancer cells arise as a result of mutations that occur in the genome of somatic cells leading to their clonal proliferation in a self-sustaining manner. Mutations lead to the formation of a new genotype, which directly shapes the cellular phenotype of tumors, which in turn differs significantly from that of normal cells. These differences are readily apparent at the level of the basal metabolism involved in energy and biomass production [1]. In normal cells, glucose is taken up by specific transporters (GLUTs—glucose transporters) and subsequently metabolized mainly via the glycolysis pathway, converting one molecule of glucose to two molecules of pyruvate, accompanied by NAD+ (Nicotinamide adenine dinucleotide, oxidized form) reduction. Assuming sufficient oxygen supply, pyruvate is then oxidized and NAD+, necessary for glycolysis, is recovered by the action of NADH (Nicotinamide adenine dinucleotide, reduced form) shuttles (translocating electrons from cytosolic to a mitochondrial pool of reducing equivalents) and the electron transport chain. It is now a well-known fact that cancer cells have a greater predisposition for lactic acid formation even under adequate oxygen supply, referred to as aerobic glycolysis, which is rather unusual for normal cells [1]. Exceptions to this dictum are mammalian cells undergoing intense proliferation, such as pluripotent stem cells, cells of the immune system, and endothelial cells, as well as regenerating tissues. For these cell types, such kind of metabolic shift was also confirmed and noted as a “hallmark of rapid proliferation” [2]. This seemingly simple metabolic change, first observed by Otto Warburg, entails a still-to-be-explored network of intra- and intercellular interactions involved in fine-tuning tumor metabolism to promote its progression. Therefore, this review aims to show the multifaceted nature of the Warburg effect, emphasizing its potential importance for the development of new therapeutic regimens enabling effective, specific, and safe treatment of cancer.
Warburg effect—a still unresolved issue
Direct effect on transformed cells
A century ago, Otto Warburg working with a rat seminal vesicle tumor unexpectedly observed a lower level of oxygen consumption by rapidly proliferating cells accompanied by high glucose uptake and lactate formation [3]. Experiments performed in various conditions in terms of glucose and/or oxygen supply led him to conclude that besides increased glucose consumption and lactate production under normal oxygen supply, oxidative metabolism is also required for cancer cell survival [4, 5]. Eventually, in contradiction to their own observations, Warburg claimed mitochondria damage as a reason for cancer transformation [6, 7]. Since then, scientists have repeatedly returned to the subject of aerobic glycolysis, named after the discoverer, the Warburg effect [8], and the last 10 years have seen a renewed interest and increasingly vigorous research into cancer cell metabolism. The aim was not only to understand the basics governing the Warburg effect, but also to comprehend the purpose of such a metabolic pathway chosen by cancer cells, and eventually exploit it therapeutically. The evolution of Warburg's idea is shown in Fig. 1. Year by year, emerging hypotheses focussing on different aspects of the Warburg effect constitute an increasingly complex puzzle, providing the insights into landscape of cancer cell metabolism from different perspectives, both as far as mechanisms and benefits are concerned. The most obvious concept indicates the need to support intensive cell divisions and shift biosynthetic precursors into anabolic reactions branching from the glycolytic pathway. This would increase the rate of nucleotide, protein, and lipid synthesis, macromolecules that are essential for intensive cell growth and proliferation [9]. The other theories postulate the existence of a glucose-deficient tumor microcosm, which accounts for the competition of the tumor cells with the T cells for nutrients and in turn explains the rapid rate of glucose uptake, in parallel resulting with immunosuppression [10]. Emphasis was also laid on the necessity of a readily available source of regenerated NAD+, which is not only necessary for the continuity of glycolysis but also required as a coenzyme for oxidized biomass synthesis [11]. Another explanation of Warburg-type metabolic alteration is the “solvent capacity limitation” theory. It states that the physical volume of the cell is insufficient to accommodate an adequately large number of mitochondria that are required to meet the enormous energy demands of the tumor [12, 13]. According to it, the citric acid cycle (TCA) is a key metabolic center responsible for supporting tumor growth [14, 15]. The “metabolic plasticity” theory allows precise switching between efficient phosphorylation and glycolysis, which is an adaptation of the tumor cell to live in different microenvironments, thereby questioning Warburg hypothesis of mitochondrial damage [16]. Consistent with the above, there is clear evidence that glycolysis is elevated in most tumors without mitochondrial dysfunction. Intensively proliferating tumor cells have an increased demand for ATP (Adenosine triphosphate) to meet the demand for metabolic reactions related to growth and cellular needs for ATP-dependent membrane pumps, e.g., Na+/K+ ATPase pump. Recent studies have correspondingly reported that changes in the cellular environment entail intensive aerobic glycolysis at a constant rate of oxidative phosphorylation to meet this demand. This provides metabolic flexibility in a high-energy demand situation for the cell [17]. On the other hand, some studies have proven that the Warburg effect can just as well be caused by mutation of the mitochondrial genes coding fumarate hydratase, succinate dehydrogenase, and isocitrate dehydrogenase necessary for TCA to take place, as well as overproduction of reactive oxygen species (ROS) by mitochondria [8, 18].
Involvement of tumor niche
In the context of increased ROS formation, the simplistic Warburg effect model evolved into the so-called reverse Warburg effect [19]. It assumes close metabolic cooperation between activated fibroblasts of the stroma and the tumor cells, demonstrating that tumor cells mainly respire aerobically, using the lactate obtained from aerobic glycolysis occurring in tumor stromal fibroblasts [19]. The ROS released from the tumor cells reciprocally induces oxidative stress in the stromal fibroblasts. It leads to HIF-1α (hypoxia-inducible factor 1-alpha) activation and further glycolysis enhancement in the stromal fibroblasts. Thus, through oxidative stress and numerous catabolic processes such as autophagy, mitophagy, and fermentation (the process of conversion of a single molecule of glucose into two lactate molecules), aerobic glycolysis leads to the formation of lactate, an oncometabolite providing a favourable environment for tumor growth and proliferation [16]. On the other hand, rapid cell division results in hypoxia in a tumor microenvironment, where oxygen demand exceeds its supply. Additionally, the presence of hypoxic niches correlates with abnormal blood vasculature, the formation and collapse of which result in hypoxia/re-oxygenation cycles. Consequently, the tumor center is relatively more hypoxic and predominantly glycolysis dependent, while the more vascularized tumor periphery relies on mitochondrial respiration in accordance with the “metabolic plasticity” theory. Moreover, these two dissimilar tumor cell populations may be metabolically linked, and substrates from different cancer cell populations may be shared and utilized. These dynamic changes of hypoxia/reoxygenation cycles induce oxidative stress, and the resulting ROS may induce the previously mentioned changes in CAFs (cancer-associated fibroblasts), leading to their metabolic reprogramming towards aerobic glycolysis [20,21,22].
Mechanism of Warburg effect
Signal transduction pathways
Undoubtedly, Otto Warburg was the first to demonstrate that one of the characteristic features of cancer cells is the utilization of the aerobic glycolysis pathway for glucose metabolism [3]. The Warburg effect is inherently associated with extensive glucose uptake and metabolism, which the cancer cell achieves through metabolic reprogramming. Metabolic reprogramming is primarily the consequence of aberrant expression of transcription factors such as HIF-1, c-MYC (cellular myelocytomatosis oncogene), p53 (protein,P53) and the PI3K/Akt/mTOR (phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin) signaling pathways [8, 23, 24]. mTOR is a serine/threonine kinase activated by various oncogenic signaling pathways and thus is overactive in cancer cells. mTOR is present as two multiprotein complexes, mTORC1 (mechanistic target of rapamycin complex 1) and m,TORC2, and is regulated by the PI3K/Akt signaling pathway, which integrates growth factor signaling with tumor cell metabolism, as well as the LKB1/AMPK (liver Kinase B1/5′AMP-activated protein kinase) pathway, controlling the energy status of the cell [25, 26]. mTOR amplifies the Warburg effect by stimulating the normoxic upregulation of the HIF-1 transcription factor among others.
HIF-1—c-Myc interplay in normoxia
HIF-1 is a heterodimer composed of a constitutively expressed beta subunit and an oxygen-dependent alpha subunit. Under conditions of reduced partial pressure of oxygen, the alpha subunit is stabilized and the HIF-1 factor as a heterodimer is translocated to the cell nucleus, where it stimulates the expression of genes coding for glycolytic enzymes and glucose transporters [27]. Among downstream mTOR effectors, is the transcription factor c-MYC. In normal cells, c-MYC is inhibited by HIF-1, but in cancer cells it interacts with HIF-1, further enhancing the Warburg effect by increasing the expression of glycolytic enzymes [28]. However, the most notable mechanism is the stimulation of the Warburg effect by mTOR/HIF-1/MYC through the upregulation of PKM2 (pyruvate Kinase M2) expression [29]. It has been shown that genetic manipulations enabling the switch of expression from PKM2 to PKM1 can effectively reverse the Warburg effect. Therefore, it seems that PKM2 expression is crucial for promoting aerobic glycolysis [30]. PKM2 is a protein present in embryonic cells, normal proliferating cells, and cancer cells. PKM2 stimulates tumor growth through dual function, acting as a glycolytic enzyme in the cytosol and as a protein kinase in the cell nucleus, leading to enhanced expression of many proteins. In cancer cells, PKM2 is present in the cytosol in the form of a dimer with low catalytic activity, which allows it to direct glycolytic intermediates on the path of anabolic transformations [31]. In addition, PKM2 interacts with the alpha subunit of HIF-1 to stimulate further metabolic reprogramming [32]. Moreover, studies have shown that pyruvate and to a lesser extent, lactate, also lead to the stabilization of the alpha subunit of HIF-1, implying the existence of a positive feedback loop [33, 34]. Thus, from the metabolic viewpoint, intensive glycolysis will lead, via the transcription factor HIF-1, to the increased expression of glycolytic enzymes and glucose transporters. Additionally, the HIF-1 factor, by upregulating the expression of PDK1 (pyruvate dehydrogenase kinase isoform 1), stimulates the phosphorylation of pyruvate dehydrogenase, and thus reduces its activity, further enhancing the Warburg effect [35].
Environmental selection of Warburg phenotype cells
Undoubtedly, normoxic stabilization of the HIF-1 transcription factor constitutes a single most crucial mechanism behind the metabolic reprogramming (Warburg effect) in cancer cells. However, it should be noted that hypoxia itself may also lead to the selection of cells with the Warburg phenotype (cells undergoing aerobic glycolysis). Recent studies have shown that conditions of low glucose, low oxygen, and low pH or starvation constitute a strong selective environment for acquiring the Warburg phenotype by cancer cells, as a result of changes at the level o,f the genome, transcriptome, and epigenome [36]. On the other hand, selection in a hypoxic environment does not seem to constitute a universal/mandatory mechanism for Warburg phenotype acquisition as another study has shown that cells selected under hypoxic conditions generated more energy via mitochondrial respiration than control cells [37].
Loss of p53 drives the Warburg effect
Another piece in the puzzle is the p53 (a crucial tumor suppressor) protein, as loss of its function also plays a role in the induction of the Warburg effect. The p53 protein regulates glucose metabolism by inhibiting the expression of glucose transporters and the activity of PFK-1 (phosphofructokinase-1). The product of the TIGAR gene, controlled by p53, reduces the activity of PFK-1 by reducing the availability of its allosteric activator fructose 2,6-bisphosphate (F-2,6-P2) [38]. The p53 protein also inhibits glycolysis indirectly by increasing the expression of PTEN, an inhibitor of the PI3K/Akt pathway [35]. Thus, releasing glycolysis from the inhibitory control of the p53 protein eventually contributes to enhancing the Warburg effect.
Warburg effect—advantages for cancer cells
Rapid ATP synthesis and NAD+ regeneration
Studies have shown that glucose and glutamine are the compounds most intensively metabolized in many cancers [39, 40]. The transformation of these molecules provides, for the most part, adequate amounts of nitrogen and carbon skeletons, as well as reducing potential and free energy necessary to maintain cellular growth. Undoubtedly, the crucial benefit of the Warburg effect is an increased ATP production rate as aerobic glycolysis can generate ATP faster than the rate of oxidative phosphorylation [41]. Intensive production of lactate, on the one hand, determines the rapid recovery of NAD+ (via lactate dehydrogenase, converting pyruvate to lactate), which allows for maintaining a high rate of glucose consumption and an appropriate level of ATP for rapid proliferation. However, on the other hand, it should be noted that the relationship between the NADH formed during glycolysis and the NAD+ obtained during lactate production by lactate dehydrogenase remains equal. Therefore, if the oxidized intermediates of glycolysis are directed into other metabolic pathways, e.g. the glycine-serine-nucleotide axis, starting from oxidation of 3-phosphoglycerate to 3-phosphohydroxypyruvate, the substrate of subsequent transamination, the NADH formed in the oxidation step must be regenerated by another red/ox reaction, for example using respiratory chain upon reducing equivalent shuttling from cytoplasm into mirochondria [42] or additional source of pyruvate is used, e.g. from the metabolism of glutamine [43]. Undoubtedly, the recently published work of Vander Heiden’s group shed new light on this issue. Their research shows that the utilization of NAD+ in cancer cells may be faster than the usage of ATP. As a consequence, the energy stored in the mitochondrial proton gradient is harnessed by ATP synthase to phosphorylate ADP (adenosine diphosphate) to make ATP. The results obtained also suggest that the higher demand for NAD+ about the demand for ATP leads to a reduction in mitochondrial respiration and thus a decrease in the NAD+/NADH ratio, promoting fermentation even when oxygen is available. Indeed, inducing ATP hydrolysis in the cell, which provides ADP for ATP synthase, released NAD+ regeneration by ETC (electron transport chain) as well as proliferation with diminished dependency on fermentation [11].
By what is mentioned above, tumors have a high demand for NAD+ to synthesize biomass, thus cancer cells often have high expression of nicotinamide phosphoribosyltransferase (NAMPT), the rate-limiting enzyme, converting nicotinamide (NAM) to nicotinamide mononucleotide (NMN), which is then converted to NAD+. This pathway is crucial to cancer cells, as it converts NAM, the catabolic product of NAD+-consuming enzymes, back to NAD+ [44, 45]. In conclusion, studies by Luengo et al. have shown that the maintenance of the optimal NAD+ pool is a key condition for maintaining a high proliferative potential, and thus the growth of cancer cells [11]. Of note, regardless of the source of lactate in the tumor cell, its formation not only enables NADH to be oxidized to NAD+ but lactate itself acts as an oncometabolite influencing the tumor microenvironment several of mechanisms that will be discussed later in the article.
Biomass and NADPH production
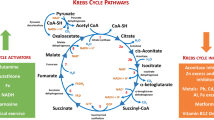
Rapid glucose metabolism besides providing energy, supplies precursors for anabolic processes, including reducing equivalents in the form of NADPH (nicotinamide adenine dinucleotide phosphate) (Fig. 2). Intermediates for the synthesis of nucleotides and non-essential amino acids are also furnished by such high glucose turnover. An example is the usage of a glycolysis intermediate, 3-phosphoglycerate, for the synthesis of serine, which in successive transformations is a donor of a one-carbon fragment for nucleotide synthesis, while at the same time being a precursor for glycine synthesis. Thus, under conditions of limited availability of serine, its de novo synthesis from the glycolysis intermediate ensures that the tumor cell maintains its proliferative potential. Another example is the use of glyceraldehyde-3-phosphate and fructose-6-phosphate, intermediates of glycolysis, in the synthesis of ribose-5-phosphate (R-5-P) via the non-oxidative arm of the pentose phosphate pathway (PPP). It should also be noted that of all the glucose that enters the cancer cell, some does not enter the glycolysis pathway at all, and is directly metabolized in the PPP oxidative arm, which is an alternative to the aforementioned R-5-P synthesis route and is additionally a source of NADPH. NADPH provides the reducing power required for the synthesis of fatty acids, sterols, nucleotides, and non-essential amino acids. Of note, if a cell requires more NADPH than a precursor for nucleotide synthesis, the excess ribulose 5-phosphate is then converted to compounds entering glycolysis in a series of reversible reactions of PPP non-oxidative arm. Thus, directing glucose into the oxidative arm of PPP does not exclude its subsequent transformation to lactate [46,47,48]. Maintaining the appropriate level of NADPH is also crucial to keep the appropriate level of reduced glutathione, protecting the cell against ROS leading to free-radical damage [46]. The concentration of ROS in cancer cells is usually high, and this promotes DNA damage and cancer progression, simultaneously having a cascading toxic effect on all cellular structures [49]. As has been shown by Anastasiou et al., increased ROS formation inhibits PKM2 isoenzyme through cysteine oxidation leading to higher glucose flux into the PPP, which in consequence decreases the oxidative stress by increasing the level of reduced glutathione [50]. Other important proteins which can be involved in glucose flow between glycolysis and the oxidative arm of PPP are tumor-specific isoenzymes of phosphofructokinase II, PFKFB3 (6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3) and PFKFB4. These proteins are highly expressed in cancer cells. It was shown that PFKFB3 acts mainly as a kinase that will promote glycolysis by stimulating the synthesis of F-2,6-BP (fructose-2,6-bisphosphate), while PFKFB4 may occur in two forms, kinase, and phosphatase. As a phosphatase, PFKFB4 hydrolyses F-2,6-BP, the glycolysis activator, and in consequence, more glucose can be directed into an alternative PPP pathway [51].
Warburg effect advantage for cancer cells—biomass and NADPH production. The main metabolic pathways contributing to biomass production in a cancer cell as nucleotide synthesis, the pentose phosphate pathway, serine synthesis, glutaminolysis, cholesterol synthesis, and fatty-acid synthesis are presented. In addition, the inhibitors of enzymes/proteins involved in the Warburg effect together with their targets are presented on the graph. ADP adenosine diphosphate, ATP adenosine triphosphate, GAPDH glyceraldehyde 3-phosphate dehydrogenase, GLUT-1 glucose transporter 1, HK-2 hexokinase-2, LDHA lactate dehydrogenase A, MCT-4 monocarboxylate transporter 4, NAD+ nicotinamide adenine dinucleotide, oxidized form, NADH nicotinamide adenine dinucleotide, reduced form, PFKFB3 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, PKM2 pyruvate kinase M2, TCA tricarboxylic acid, THF tetrahydrofolate
Lactate as an oncometabolite
The increased production of lactate associated with the Warburg effect results in a decreased pH and thus provides an acidic tumor microenvironment (TME). The lactate generated together with H+ ions, which are formed mainly in the process of converting glucose into pyruvate, are transported through the cell membrane with the help of MCT1 (monocarboxylate transporter 1) and MCT4 (monocarboxylate transporter 4) transporters, on a symport basis, a form of secondary active transport [52]. Moreover, a change in the pH in the extracellular microenvironment of the tumor stimulates its development, promoting cell spreading, and consequently leads to metastasis [53]. Tumor progression is manifested in three aspects: (1) extracellular matrix (ECM) degradation and tumor cell migration (2) angiogenesis and (3) immunosuppression [54]. In each of these aspects, lactate plays an indirect role, providing a low pH environment, a factor that directly affects tumor progression. The process of direct migration of tumor cells begins with ECM degradation. Low pH activates enzymes such as matrix metalloproteinases (MMPs) released from the tumor cell, causing digestion of the surrounding matrix—this allows the cells to detach from the solid substrate. A direct correlation between the concentration of lactate and the activity of proteinases was demonstrated for cathepsin B, hyaluronidase-2 [55], and matrix metalloproteinases-9 [56]. The next step is the migration (via lymphatic/ hematogenous/direct or trans-coelomic routes) and attachment of free cancer cells to other tissues. Due to lactic acidosis, the production of actin filaments is activated [57], the properties of integrins on the surface of cancer cells [58] are changed, the number and size of 'invadopodia' (adhesive, membrane structures, containing proteases responsible for ECM degradation) are increased [59] and the expression of hyaluronan and CD44 (cluster of differentiation 44) is increased [60]. These processes allow for the movement and adhesion of free cancer cells to healthy tissues, and at a later stage, for the development of metastases. Studies have confirmed a positive correlation between high lactate levels and metastases in cervical cancer [61], head and neck cancers [62], colorectal adenocarcinoma [56, 63] and lung cancer [64]. Moreover, lactate is suggested also as an important player in the pathogenesis of metastatic spine disease [54] and generally high lactate concentrations in cancer cells have been associated with an overall more aggressive disease course and reduced chances of survival [65]. High lactate concentration in tumor cells affects also the process of angiogenesis through two distinct pathways, depending on the individual products of the lactate dehydrogenase (LDH) reaction. In the first case, the accumulation of pyruvate leads to the inhibition of prolyl hydroxylases (PHDs), resulting in a decreased degradation of HIF-1α [66]. The HIF-1ɑ factor activates the expression of proangiogenic mediators such as bFGF (basic fibroblast growth factor), SDF-1 (Stromal cell-derived factor 1), and VEGF (Vascular endothelial growth factor) [67]. In the second case, the accumulation of pyruvate leads to the accumulation of NADH, which activates the NADPH oxidase enzyme and promotes the formation of superoxide anion radical, resulting in a cascade of subsequent effects: it leads to ROS-dependent degradation of IĸBɑ (NF-κB protein inhibitor), activation of NF-kB (Nuclear factor kappa B) and the expression of IL-8 (interleukin 8)—a pro-angiogenic cytokine [64, 68]. Lactate also impairs the immune response in the tumor microenvironment, through a variety of effects on the cells of the immune system: T-cells, natural killers (NK) and natural killer T cells (NKT), dendritic cells, and macrophages [69]. In the case of T-cells, a high concentration of lactate has been linked to the inhibition of migration and cytotoxicity for CD4+ (cluster of differentiation 4) and CD8+ (cluster of differentiation 8) cells by disrupting lactate export [69], while a decrease in NAD+ T-cells’ levels, resulting from the presence of lactate, induces their apoptosis by suppressing FIP200 (focal adhesion kinase family interacting protein of 200 kD) [70]. Lactate blocks the production of pro-inflammatory cytokines: IL-4 and IFN by NKT cells, by inhibiting the mTOR signaling pathway [71]. A high lactate-to-glucose ratio stimulates lactate-avid Treg cells, which promote immunosuppression in the tumor environment [72]. Accumulation of lactate and the associated low pH in the tumor cell environment directly inactivates NK cells, causing their apoptosis and disrupting the regulation of the nuclear factor of activated T cells (NFAT), reducing the production of IFN-y (Interferon y). Dendritic cells (DCs), responsible for antigen presentation, increase the production of the immunosuppressive cytokine IL-10 (interleukin 10) as a result of lactate accumulation and limit differentiation [73]. In the presence of lactate, the anti-tumor function of M1 macrophages is inhibited by reducing the expression of IL-6 (interleukin 6), IL–1 (interleukin 1), iNOS (inducible nitric oxide synthase), and TNF (tumor necrosis factor) [72, 74], while inactivated macrophages undergo an induced polarization process changing their phenotypes, that results in the formation of pro-tumor M2 macrophages [75, 76]. The relationship between the presence of lactate in TME and immunosuppression has been demonstrated in the following cancers: breast cancer [77], prostate carcinoma [78], cervical cancer [79], and pancreatic cancer [80]. In conclusion, lactate plays an important role as an oncometabolite in the development of cancer, generating a low pH in the tumor microenvironment and participating in metabolic symbiosis. It is responsible for the greater malignant potential of tumor cells, and therapeutic resistance, and stimulates the loss of adhesion and subsequent migration of cancer cells leading to metastasis, angiogenesis, and immunosuppression in the vicinity of the tumor. Treatments based on the modification of the tumor microenvironment are the basis for potential future anti-cancer therapies [81].
Reverse Warburg effect
Several ambiguities related to the functioning of tumor cells and, above all, the determination of scientists to solve the intricate metabolic puzzle, allowed Warburg's idea to evolve, through many different models and hypotheses, until 2009, when the so-called reverse Warburg effect was introduced into the scientific discourse [19]. According to the reverse Warburg effect hypothesis, lactate is the most important metabolic fuel for cancer cells because, through a metabolic symbiosis between cancer cells and cancer-associated fibroblasts, it enables tumor self-sufficiency for cancer cells [82].To delve into the essence of this mechanism, it's worth taking a close look at the cellular environment of cancer. All tumors have two basic components: neoplastic parenchymal cells and the associated stroma consisting of blood vessels, immune cells, and supporting cells. Their classification and biological behaviour are mainly decided by the parenchyma, whereas their growth and spread are determined by the stromal components. This microenvironmental stroma is formed by tumor-associated inflammatory cells, fibroblasts, lymphoid cells, and vascular endothelial cells. The reverse Warburg effect mechanism involves both activated fibroblasts and the parenchymal tumor cells. The differentiation of stromal cells into CAFs takes place in the extracellular compartment of the tumor under the influence of tumor cells [19]. The tumor cells disturb the physiological stroma and turn it into a factory of energy-rich metabolites [19]. Tumor cells also lead to oxidative stress on the fibroblasts by generating ROS in the form of H2O2 [83], resulting in numerous catabolic processes in the cells, such as autophagy, mitophagy, and lactic acid fermentation [84, 85].
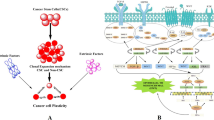
It is here that the role of a factory of energy-rich metabolites is revealed, as through these processes, CAFs provide cancer cells with large amounts of lactate, ketones, or glutamine, which are fuel for bio-synthetic reactions, numerous anabolic processes, and the production of large amounts of ATP [86, 87]. Thus, it would be correct to view the reverse Warburg effect as a parasitic metabolic pathway in which cancer cells have their metabolic source in the CAFs while generating ROS and creating conditions for the production of lactate by other cells. The pressure that tumors exert on fibroblasts gives them the phenotype of the Warburg effect proper—fibroblasts are the site of intensive aerobic glycolysis, while tumor cells generally respire normally and draw only pyruvate and lactate from CAFs. Therefore, the reverse Warburg effect is a two-compartment model showing metabolic symbiosis or metabolic coupling between CAFs and tumor cells. This mechanism is thus an alternative to the “ancestral mutation model,” as epithelial tumor cells instruct stromal cells to transform into the energy-rich stroma, thereby facilitating tumor growth and angiogenesis [19, 82, 88]. Glucose is the most important metabolite used by the neoplastic cells for ATP generation, production of essential cellular components and regulation of the redox state of cells [9]. Its catabolism, or aerobic glycolysis, is predominantly in the CAFs/ stromal fibroblasts that generate large quantities of lactate through it [82]. Lactate is consequently secreted from the cytoplasm of CAFs into the extracellular space. Normally, this entails the expression of the monocarboxyl transporters, MCT4 and MCT1 [88]. Most studies indicate that MCT4 has the lowest affinity for lactate (high Km) among MCTs and facilitates lactic acid efflux from glycolytic cells, including hypoxic cancer cells. MCT1, on the other hand, has a high affinity for lactate (low Km) and primarily mediates lactic acid influx, used subsequently as an oxidative fuel for mitochondrial respiration [89]. Of note, MCT4, as opposed to MCT1, is up-regulated by hypoxia, through a HIF-1- dependent mechanism, promoting lactate efflux from hypoxic cells [90]. High expression of both, MCT1 and MCT4, is observed in many tumors which usually correlates with poorer overall survival [89]. However, in triple-negative breast cancer and in non-small cell lung cancer, high expression of only MCT4, not MCT1, correlated with a worse prognosis [91, 92]. Understandably, these transporters are overexpressed also in CAFs [93]. Accumulated lactate, other secretions from tumor cells and oxidative stress cumulatively create conditions, which differentiate tumor cells into two major subsets: hypoxic tumor cells and oxidative tumor cells, and the cause of their intrinsic metabolic heterogeneity [82, 94]. Hypoxic tumor cells direct their metabolism toward anaerobic glycolysis, generating lactate, as in CAFs [93, 95]. The metabolites mainly lactate and small amounts of pyruvate are then exported from the cytoplasm of the cells to the extracellular space by the previously mentioned specific MCT4 transporters [19]. Thus, the lactate generated by these cells and CAFs provides a huge and ready pool of fuel for neighboring tumor cells to meet their metabolic demands [96]. Its transport into the interior of these cells is possible via MCT-1 transporters, which are the main importers of lactate [97, 98]. Such metabolic flexibility owes its existence to an efficient lactate shuttle (Fig. 3). Lactate that is not utilized in this way is deposited in the extra-cellular space and results in the formation of an acidic microenvironment, a condition that further promotes carcinogenesis [52]. Unused lactate is metabolized by oxidative phosphorylation, resulting in the generation of ATP in normoxic tumor cells. Importantly, the vast majority of cells in the tumor microenvironment are oxidative cells, which, according to the presumptions of the reverse Warburg effect, generate the greatest amount of energy in the form of ATP, which is essential for tumor growth, proliferation, progression, and most importantly, metastasis. To achieve it, they use a bidirectional enzyme—lactate dehydrogenase responsible for the conversion of lactate back to pyruvate, which enables its subsequent incorporation into the Krebs cycle and ultimate energy acquisition via the oxidative pathway [99]. It is worth noting that LDH consists of two subunits (M) and (H) codified by the LDHA and LDHB genes, respectively. These subunits can form 5 different combinations of homo and heterodimers LDH-1 (4H), LDH-2 (3H1M), LDH-3 (2H2M), LDH-4 (1H3M) and LDH-5 (4 M). In cancer cells, LDH-1 and LDH-5 are preferentially expressed. The LDH-5, coded by the LDHA gene preferentially converts pyruvate to lactate, whereas the LDH-1, coded by the LDHB gene, opposite, lactate into pyruvate. Thus, lactate-forming cells will have higher expression of LDHA, whereas lactate-consuming cells will preferentially express the LDHB gene [100]. As high expression of LDHA and/or LDHB is associated with poor prognosis, these enzymes, as well as MCT1 and MCT4 lactate transporters, can serve as important therapeutic targets for anti-cancer therapy. Interestingly, a recent study, analyzing metabolic signature in human cancers at the single-cell level, revealed the coexistence of glycolytic and mitochondrial metabolic signature among cells in the same tumor, confirming “metabolic plasticity” and indicating the reverse Warburg effect between different cancer cell subpopulations [101]. Thus, targeting a protein/proteins associated with different metabolic profiles seems to be an optimal approach for an anti-cancer strategy.
Reverse Warburg effect. In the reverse Warburg effect, oxidative cancer cells can take up lactate from hypoxic cancer cells. Moreover, oxidative cancer cells induce oxidative stress in cancer-associated fibroblasts (CAFs) by secreting reactive oxygen spiecies (ROS), which in turn triggers the aerobic glycolysis in CAFs. In consequence, lactate and pyruvate produced by CAFs are metabolized in adjacent oxidative cancer cells. AC-CoA acetyl coenzyme A
Warburg effect—as a potential therapeutic target
Given the multifaceted nature of the Warburg effect—both in terms of causes and benefits for the transformed cell, it seems an obvious target in new therapeutic regimens. However, the question arises—which dominoes to hit to “collapse” the cancer? Recent years have shown that the Warburg effect may not only be about the rapid generation of ATP and directing intermediates to biosynthetic pathways, but it may also result from the too-slow recovery of NAD+ in relation to the rate of energy generation in the form of ATP. In such a situation, it is cost-effective for the cell to regenerate NAD+ by promoting fermentation. Another issue is the reverse Warburg effect, which itself could become a therapeutic target as well. The very initiation of the reverse Warburg effect is a consequence of the production of ROS, so reducing the oxidative stress in cancer cells could lead to the inhibition of the reverse Warburg effect. Thereby, another domino cubes come here, the enzymes PFKFB3 and PFKFB4, which direct the flow of glucose between the PPP pathway and glycolysis. However, to become an important therapeutic target, the catalytic function of these enzymes would have to be well recognized in particular cancer. According to the latest data, it is the expression at the level of these isoforms to determine whether phosphatase activity will dominate [102], which directs glucose to PPP increasing the same protection against ROS, or kinase activity, accelerating the glycolysis pathway. Another issue is lactate acting as an oncometabolite mediating migration, invasion, immunosuppression, and angiogenesis. It would seem that the inhibition of LDH will not only inhibit the regeneration of NAD+, but also the production of this significant cancer metabolite, and will prevent the use of lactate as an energy fuel in the reverse Warburg effect. On the other hand, many normal cells of the immune system rely on aerobic glycolysis, and such intervention would still promote tumor-promoting immunosuppression. A more effective and tumor-selective intervention in the reprogrammed metabolism of cancer cells would be the use of the lactate-consuming enzyme; lactate oxidase, an approach that, especially in the form of targeted nanoparticles, should bring more benefits and fewer side effects [103]. Table 1 presents inhibitors of selected proteins involved in the Warburg effect, currently under investigation for treating cancer. Some of these compounds entered the clinical phase of trials, showing an inhibitory effect on tumor growth, as revealed by recent studies. Despite some issues concerning drug delivery and bioavailability, specificity, and toxicity, cancer cell plasticity, and heterogeneity of tumor cells that make it difficult to apply monotherapy schemes, glucose metabolism inhibitors still have anticancer potential as components of multi-agent regimens [24, 104]. The reader can find much more detailed information in the latest publications devoted to the inhibition of anaerobic glycolysis in the context of improving some of the currently used anticancer therapies. Numerous basic preclinical research has focused on multiple Warburg effect inhibitors, as single agents or in combinations, but few of them have entered clinical trials [104, 105]. Among them there are 2-deoxy glucose (hexokinase inhibitor, tested alone or in combination with docetaxel in patients with advanced solid tumors) [106], lonidamine (hexokinase inhibitor, tested in combination with various chemotherapeutic agents, e.g. 5-fluorouracil, doxorubicin, cisplatin in several advanced cancer) [107], indisulam (carbonic anhydrase inhibitor, tested in combination with capecitabine/Irinotecan in metastatic colorectal cancer) [108] and AZD3965 (MCT1 inhibitor, tested alone in some lymphomas and solid tumors) [104]. To date, none of the tested compounds, alone or in combination, have brought satisfactory results allowing for use in routine clinical practice. Nevertheless, one should be aware that in the era of constantly developing theranostic and increasingly advanced research possibilities, it is more than likely that the general findings made on cancer models (cell lines, animals, groups of selected cancer patients) may not be applicable to large cohorts of patients, but could evince great importance in the individualized approach to individual patients.
Concluding remarks
Despite researchers’ efforts, cancer cell metabolism still eludes a full understanding and clarification of the complex interconnections in terms of intracellular, cancer cell-cancer cell, and cancer cell-tumor stroma interactions. The seemingly simple metabolic reprogramming observed in transformed cells by Otto Warburg about a century ago, later recognized as one of the main hallmarks of cancer, is even now an area of detailed investigation, gradually uncovering the complexity of tumor functioning. Behind this effect of increased “aerobic glycolysis”, leading to lactate production despite normal oxygen supply, is a dysregulated intracellular signaling, enhanced biomass production potential, efficient energy supply as well as the increased metastatic ability or immunosuppression. In concert with intratumor interrelationship, where populations with different metabolic activities (based on glycolysis or oxidative phosphorylation) coexist, cancer cells take advantage of cancer-associated fibroblasts by first forcing their metabolic reprogramming and then consuming its products. In view of the presented facts and hypotheses regarding metabolic reprogramming in cancer cells, it is more than obvious that the more closely this phenomenon is studied, the more metabolic connections emerge. The issue seems even more intricate as what was previously considered the main benefit of the Warburg effect for cancer cells may no longer be so in light of recent research. Therefore, only a better understanding of the mechanisms of metabolic alterations and their interrelations can allow for an accurate and effective hit in the changed metabolism, which undoubtedly determines the rapid proliferation and tumor biomass production. Clearly, targeting specific nodal points regulating metabolite flux in tumors rather than targeting individual glycolytic enzymes should bring the desired effects in terms of specificity and treatment efficacy.
Data availability
The submitted manuscript has no associated data.
Abbreviations
- ADP:
-
Adenosine diphosphate
- Akt:
-
Protein kinase B
- AMPK:
-
5′AMP-activated protein kinase
- ATP:
-
Adenosine triphosphate
- BFGF:
-
Basic fibroblast growth factor
- CAFs:
-
Cancer-associated fibroblasts
- CD4+:
-
Cluster of differentiation 4
- CD44:
-
Cluster of differentiation 44
- CD8+:
-
Cluster of differentiation 8
- c-Myc:
-
Cellular myelocytomatosis oncogene
- DCs:
-
Dendritic cells
- ECM:
-
Extracellular matrix
- ETC:
-
Electron transport chain
- F-2,6-BP:
-
Fructose-2,6-bisphosphate
- F-2,6-P2:
-
Fructose 2,6-bisphosphate
- FIP200:
-
Focal adhesion kinase family interacting protein of 200 kD
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GLUTs:
-
Glucose transporters
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- IFN-γ:
-
Interferon γ
- IkBα:
-
NF-κB protein inhibitor
- IL-1:
-
Interleukin 1
- IL-10:
-
Interleukin 10
- IL-6:
-
Interleukin 6
- IL-8:
-
Interleukin 8
- iNOS:
-
Inducible nitric oxide synthase
- LDH:
-
Lactate dehydrogenase
- LKB1:
-
Liver kinase B1
- MCT1:
-
Monocarboxylate transporter 1
- MCT4:
-
Monocarboxylate transporter 4
- MMPs:
-
Matrix metalloproteinases
- mTOR:
-
Mammalian target of rapamycin
- mTORC1/2:
-
Mechanistic target of rapamycin complex 1/2
- NAD+:
-
Nicotinamide adenine dinucleotide, oxidized form
- NADH:
-
Nicotinamide adenine dinucleotide, reduced form
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NAM:
-
Nicotinamide
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- NFAT:
-
Nuclear factor of activated T-cells
- NF-kB:
-
Nuclear factor kappa B
- NKT:
-
Natura killer T cells
- NMN:
-
Nicotinamide mononucleotide
- p53:
-
Protein P53
- PDK1:
-
Phosphoinositide-dependent kinase-1
- PFK1:
-
Phosphofructokinase-1
- PFKFB3/4:
-
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3/4
- PHDs:
-
Prolyl hydroxylases
- PI3K:
-
Phosphoinositide 3-kinase
- PKM2/1:
-
Pyruvate kinase M2/M1
- PPP:
-
Pentose phosphate pathway
- R-5-P:
-
Ribose-5-phosphate
- ROS:
-
Reactive oxygen species
- SDF-1:
-
Stromal cell-derived factor 1
- TME:
-
Tumor microenvironment
- TNF:
-
Tumor necrosis factor
- VEGF:
-
Vascular endothelial growth factor
References
DeBerarinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5): e1600200.
Abdel-Haleem AM, Lewis NE, Jamshidi N, Mineta K, Gao X, Gojobori T. The emerging facets of non-cancerous Warburg effect. Front Endocrinol (Lausanne). 2017;8:279.
Warburg O. The metabolism of carcinoma cells. J Cancer Res. 1925;9(1):148–63.
Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12(50):1131–7.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–30.
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14.
Warburg O. On respiratory impairment in cancer cells. Science. 1956;124(3215):269–70.
Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599(6):1745–57.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33.
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41.
Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell. 2021;81(4):691-707.e6.
Vazquez A, Liu J, Zhou Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;6(4):58.
Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–80.
Bonekamp NA, Peter B, Hillen HS, Felser A, Bergbrede T, Choidas A, et al. Small-molecule inhibitors of human mitochondrial DNA transcription. Nature. 2020;588(7839):712–6.
Martínez-Reyes I, Cardona LR, Kong H, Vasan K, McElroy GS, Werner M, et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature. 2020;585(7824):288–92.
Schiliro C, Firestein BL. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 2021;10(5):1056.
Epstein T, Xu L, Gillies RJ, Gatenby RA. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab. 2014;2:7.
Ghanbari Movahed Z, Rastegari-Pouyani M, Mohammadi MH, Mansouri K. Cancer cells change their glucose metabolism to overcome increased ROS: one step from cancer cell to cancer stem cell? Biomed Pharmacother. 2019;112: 108690.
Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8(23):3984–4001.
Nakajima EC, Van Houten B. Metabolic symbiosis in cancer: refocusing the Warburg lens. Mol Carcinog. 2013;52(5):329–37.
Bader SB, Dewhirst MW, Hammond EM. Cyclic hypoxia: an update on its characteristics, methods to measure it and biological implications in cancer. Cancers (Basel). 2020;13(1):23.
Roy S, Kumaravel S, Sharma A, Duran CL, Bayless KJ, Chakraborty S. Hypoxic tumor microenvironment: implications for cancer therapy. Exp Biol Med (Maywood). 2020;245(13):1073–86.
Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med (Berl). 2011;89(3):221–8.
Chen XS, Li LY, Guan YD, Yang JM, Cheng Y. Anticancer strategies based on the metabolic profile of tumor cells: therapeutic targeting of the Warburg effect. Acta Pharmacol Sin. 2016;37(8):1013–9.
Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–75.
Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36(16):2191–201.
Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007(407):cm8.
Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8(1):51–6.
Sun Q, Chen X, Ma J, Peng H, Wang F, Zha X, et al. Mammalian target of rapamycin up-regulation of pyruvate kinase isoenzyme type M2 is critical for aerobic glycolysis and tumor growth. Proc Natl Acad Sci USA. 2011;108(10):4129–34.
Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3.
Zahra K, Dey T, Ashish, Mishra SP, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol. 2020;10:159.
Azoitei N, Becher A, Steinestel K, Rouhi A, Diepold K, Genze F, et al. PKM2 promotes tumor angiogenesis by regulating HIF-1α through NF-κB activation. Mol Cancer. 2016;6(15):3.
Jung SY, Song HS, Park SY, Chung SH, Kim YJ. Pyruvate promotes tumor angiogenesis through HIF-1-dependent PAI-1 expression. Int J Oncol. 2011;38(2):571–6.
Sonveaux P, Copetti T, De Saedeleer CJ, Végran F, Verrax J, Kennedy KM, et al. Targeting the lactate transporter MCT1 in endothelial cells inhibits lactate-induced HIF-1 activation and tumor angiogenesis. PLoS ONE. 2012;7(3): e33418.
Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–85.
Damaghi M, West J, Robertson-Tessi M, Xu L, Ferrall-Fairbanks MC, Stewart PA, et al. The harsh microenvironment in early breast cancer selects for a Warburg phenotype. Proc Natl Acad Sci USA. 2021. https://doi.org/10.1073/pnas.2011342118.
Verduzco D, Lloyd M, Xu L, Ibrahim-Hashim A, Balagurunathan Y, Gatenby RA, et al. Intermittent hypoxia selects for genotypes and phenotypes that increase survival, invasion, and therapy resistance. PLoS ONE. 2015;10(3): e0120958.
Lee P, Vousden KH, Cheung EC. TIGAR, TIGAR, burning bright. Cancer Metab. 2014;2(1):1.
Hay N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat Rev Cancer. 2016;16(10):635–49.
Jin J, Byun JK, Choi YK, Park KG. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp Mol Med. 2023;55(4):706–15.
Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–7.
Vaupel P, Mayer A. Availability, not respiratory capacity governs oxygen consumption of solid tumors. Int J Biochem Cell Biol. 2012;44(9):1477–81.
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104(49):19345–50.
Heske CM. beyond energy metabolism: exploiting the additional roles of NAMPT for cancer therapy. Front Oncol. 2019;9:1514.
Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–41.
Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2014;39(8):347–54.
Kanarek N, Petrova B, Sabatini DM. Dietary modifications for enhanced cancer therapy. Nature. 2020;579(7800):507–17.
Geeraerts SL, Heylen E, De Keersmaecker K, Kampen KR. The ins and outs of serine and glycine metabolism in cancer. Nat Metab. 2021;3(2):131–41.
Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203.
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334(6060):1278–83.
Yi M, Ban Y, Tan Y, Xiong W, Li G, Xiang B. 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 and 4: a pair of valves for fine-tuning of glucose metabolism in human cancer. Mol Metab. 2019;20:1–13.
Corbet C, Feron O. Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer. 2017;17(10):577–93.
Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13(1):89.
Pennington Z, Goodwin ML, Westbroek EM, Cottrill E, Ahmed AK, Sciubba DM. Lactate and cancer: spinal metastases and potential therapeutic targets (part 2). Ann Transl Med. 2019;7(10):221.
Witschen PM, Chaffee TS, Brady NJ, Huggins DN, Knutson TP, LaRue RS, et al. Tumor cell associated hyaluronan-CD44 signaling promotes pro-tumor inflammation in breast cancer. Cancers (Basel). 2020;12(5):1325.
Putney LK, Barber DL. Expression profile of genes regulated by activity of the Na-H exchanger NHE1. BMC Genomics. 2004;5(1):46.
Peppicelli S, Bianchini F, Calorini L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev. 2014;33(2–3):823–32.
Paradise RK, Lauffenburger DA, Van Vliet KJ. Acidic extracellular pH promotes activation of integrin α(v)β(3). PLoS ONE. 2011;6(1): e15746.
Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. FASEB J. 2010;24(10):3903–15.
Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res. 2002;276(1):24–31.
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60(4):916–21.
Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51(2):349–53.
Walenta S, Chau TV, Schroeder T, Lehr HA, Kunz-Schughart LA, Fuerst A, et al. Metabolic classification of human rectal adenocarcinomas: a novel guideline for clinical oncologists? J Cancer Res Clin Oncol. 2003;129(6):321–6.
Vlachostergios PJ, Oikonomou KG, Gibilaro E, Apergis G. Elevated lactic acid is a negative prognostic factor in metastatic lung cancer. Cancer Biomark. 2015;15(6):725–34.
Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Curr Med Chem. 2004;11(16):2195–204.
Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280(51):41928–39.
Dhup S, Dadhich RK, Porporato PE, Sonveaux P. Multiple biological activities of lactic acid in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des. 2012;18(10):1319–30.
Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206: 107451.
Wang ZH, Peng WB, Zhang P, Yang XP, Zhou Q. Lactate in the tumour microenvironment: from immune modulation to therapy. EBioMedicine. 2021;73: 103627.
Xia H, Wang W, Crespo J, Kryczek I, Li W, Wei S, et al. Suppression of FIP200 and autophagy by tumor-derived lactate promotes naïve T cell apoptosis and affects tumor immunity. Sci Immunol. 2017;2(17):eaan4631.
Kumar A, Pyaram K, Yarosz EL, Hong H, Lyssiotis CA, Giri S, et al. Enhanced oxidative phosphorylation in NKT cells is essential for their survival and function. Proc Natl Acad Sci USA. 2019;116(15):7439–48.
Multhoff G, Vaupel P. Lactate-avid regulatory T cells: metabolic plasticity controls immunosuppression in tumour microenvironment. Signal Transduct Target Ther. 2021;6(1):171.
Nasi A, Fekete T, Krishnamurthy A, Snowden S, Rajnavölgyi E, Catrina AI, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol. 2013;191(6):3090–9.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63.
Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;15(877): 173090.
Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA. 2017;114(3):580–5.
Comito G, Iscaro A, Bacci M, Morandi A, Ippolito L, Parri M, et al. Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38(19):3681–95.
Stone SC, Rossetti RAM, Alvarez KLF, Carvalho JP, Margarido PFR, Baracat EC, et al. Lactate secreted by cervical cancer cells modulates macrophage phenotype. J Leukoc Biol. 2019;105(5):1041–54.
Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol. 2013;191(3):1486–95.
Wang JJ, Lei KF, Han F. Tumor microenvironment: recent advances in various cancer treatments. Eur Rev Med Pharmacol Sci. 2018;22(12):3855–64.
Benny S, Mishra R, Manojkumar MK, Aneesh TP. From Warburg effect to reverse Warburg effect; the new horizons of anti-cancer therapy. Med Hypotheses. 2020;144: 110216.
Chan JS, Tan MJ, Sng MK, Teo Z, Phua T, Choo CC, et al. Cancer-associated fibroblasts enact field cancerization by promoting extratumoral oxidative stress. Cell Death Dis. 2017;8(1): e2562.
Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, et al. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle. 2010;9(16):3256–76.
de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143.
Bonuccelli G, Whitaker-Menezes D, Castello-Cros R, Pavlides S, Pestell RG, Fatatis A, et al. The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle. 2010;9(10):1960–71.
Whitaker-Menezes D, Martinez-Outschoorn UE, Flomenberg N, Birbe RC, Witkiewicz AK, Howell A, et al. Hyperactivation of oxidative mitochondrial metabolism in epithelial cancer cells in situ: visualizing the therapeutic effects of metformin in tumor tissue. Cell Cycle. 2011;10(23):4047–64.
Johar D, Elmehrath AO, Khalil RM, Elberry MH, Zaky S, Shalabi SA, et al. Protein networks linking Warburg and reverse Warburg effects to cancer cell metabolism. BioFactors. 2021;47(5):713–28.
Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48–66.
Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem. 2006;281(14):9030–7.
Doyen J, Trastour C, Ettore F, Peyrottes I, Toussant N, Gal J, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun. 2014;451(1):54–61.
Tong YH, Hu XP, Xiang XP, Fang L. High expression of monocarboxylate transporter 4 (MCT 4), but not MCT 1, predicts poor prognosis in patients with non-small cell lung cancer. Transl Cancer Res. 2021;10(3):1336–45.
Crisp A, Verlengia R, Luiz da Rocha G, Mota G, Pellegrinotti Í, Lopes C. Lactate and monocarboxylate transporters (MCTs): a review of cellular aspects. J Exerc PhysiolOnline. 2015;18:1–13.
Kim J, DeBerardinis RJ. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 2019;30(3):434–46.
Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;25(5): e190.
Li F, Simon MC. Cancer cells don’t live alone: metabolic communication within tumor microenvironments. Dev Cell. 2020;54(2):183–95.
Fu Y, Liu S, Yin S, Niu W, Xiong W, Tan M, et al. The reverse Warburg effect is likely to be an Achilles’ heel of cancer that can be exploited for cancer therapy. Oncotarget. 2017;8(34):57813–25.
Avagliano A, Granato G, Ruocco MR, Romano V, Belviso I, Carfora A, et al. Metabolic reprogramming of cancer associated fibroblasts: the slavery of stromal fibroblasts. Biomed Res Int. 2018;2018:6075403.
Goodwin ML, Gladden LB, Nijsten MW, Jones KB. Lactate and cancer: revisiting the Warburg effect in an era of lactate shuttling. Front Nutr. 2014;1:27.
Mishra D, Banerjee D. Lactate dehydrogenases as metabolic links between tumor and stroma in the tumor microenvironment. Cancers (Basel). 2019;11(6):750.
Xiao Z, Dai Z, Locasale JW. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat Commun. 2019;10(1):3763.
Trojan SE, Dudzik P, Totoń-Żurańska J, Laidler P, Kocemba-Pilarczyk KA. Expression of alternative splice variants of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 in normoxic and hypoxic melanoma cells. Int J Mol Sci. 2021;22(16):8848.
Choi H, Yeo M, Kang Y, Kim HJ, Park SG, Jang E, et al. Lactate oxidase/catalase-displaying nanoparticles efficiently consume lactate in the tumor microenvironment to effectively suppress tumor growth. J Nanobiotechnol. 2023;21(1):5.
Kozal K, Jóźwiak P, Krześlak A. Contemporary perspectives on the Warburg effect inhibition in cancer therapy. Cancer Control. 2021;28:10732748211041244.
Chelakkot C, Chelakkot VS, Shin Y, Song K. Modulating glycolysis to improve cancer therapy. Int J Mol Sci. 2023;24(3):2606.
Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, et al. A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(2):523–30.
Cervantes-Madrid D, Romero Y, Dueñas-González A. Reviving lonidamine and 6-diazo-5-oxo-l-norleucine to be used in combination for metabolic cancer therapy. Biomed Res Int. 2015;2015: 690492.
Zhong X, He X, Wang Y, Hu Z, Huang H, Zhao S, et al. Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J Hematol Oncol. 2022;15(1):160.
Yakisich JS, Azad N, Kaushik V, Iyer AKV. The biguanides metformin and buformin in combination with 2-deoxy-glucose or WZB-117 inhibit the viability of highly resistant human lung cancer cells. Stem Cells Int. 2019;2019:6254269.
Reckzeh ES, Waldmann H. Small-molecule inhibition of glucose transporters GLUT-1-4. ChemBioChem. 2020;21(1–2):45–52.
Ceballos J, Schwalfenberg M, Karageorgis G, Reckzeh ES, Sievers S, Ostermann C, et al. Synthesis of indomorphan pseudo-natural product inhibitors of glucose transporters GLUT-1 and -3. Angew Chem Int Ed Engl. 2019;58(47):17016–25.
Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson JS, Kelly K, et al. A phase I dose-finding study of silybin phosphatidylcholine (milk thistle) in patients with advanced hepatocellular carcinoma. Integr Cancer Ther. 2014;13(1):46–53.
Kumthekar P, Ko CH, Paunesku T, Dixit K, Sonabend AM, Bloch O, et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci Transl Med. 2021. https://doi.org/10.1126/scitranslmed.abb3945.
Dwarakanath BS, Singh D, Banerji AK, Sarin R, Venkataramana NK, Jalali R, et al. Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects. J Cancer Res Ther. 2009;5(Suppl 1):S21–6.
Huang Y, Sun G, Sun X, Li F, Zhao L, Zhong R, et al. The potential of lonidamine in combination with chemotherapy and physical therapy in cancer treatment. Cancers (Basel). 2020;12(11):3332.
Roehrborn CG. The development of lonidamine for benign prostatic hyperplasia and other indications. Rev Urol. 2005;7(Suppl 7):S12-20.
Li J, Pan J, Liu Y, Luo X, Yang C, Xiao W, et al. 3-Bromopyruvic acid regulates glucose metabolism by targeting the c-Myc/TXNIP axis and induces mitochondria-mediated apoptosis in TNBC cells. Exp Ther Med. 2022;24(2):520.
Nilsson H, Lindgren D, Mandahl Forsberg A, Mulder H, Axelson H, Johansson ME. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-bromopyruvate. Cell Death Dis. 2015;6(1): e1585.
Jeoung NH, Jo AL, Park HS. Synergistic effects of autocrine motility factor and methyl jasmonate on human breast cancer cells. Biochem Biophys Res Commun. 2021;18(558):22–8.
Zheng M, Wu C, Yang K, Yang Y, Liu Y, Gao S, et al. Novel selective hexokinase 2 inhibitor benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol Res. 2021;164: 105367.
Wang Z, Lv J, Li X, Lin Q. The flavonoid astragalin shows anti-tumor activity and inhibits PI3K/AKT signaling in gastric cancer. Chem Biol Drug Des. 2021;98(5):779–86.
Ren B, Kwah MX, Liu C, Ma Z, Shanmugam MK, Ding L, et al. Resveratrol for cancer therapy: challenges and future perspectives. Cancer Lett. 2021;1(515):63–72.
Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160(5):714–7.
Byun WS, Bae ES, Park SC, Kim WK, Shin J, Lee SK. Antitumor activity of asperphenin B by induction of apoptosis and regulation of glyceraldehyde-3-phosphate dehydrogenase in human colorectal cancer cells. J Nat Prod. 2021;84(3):683–93.
Jing C, Li Y, Gao Z, Wang R. Antitumor activity of koningic acid in thyroid cancer by inhibiting cellular glycolysis. Endocrine. 2022;75(1):169–77.
Reda A, Refaat A, Abd-Rabou AA, Mahmoud AM, Adel M, Sabet S, et al. Role of mitochondria in rescuing glycolytically inhibited subpopulation of triple negative but not hormone-responsive breast cancer cells. Sci Rep. 2019;9(1):13748.
Lee J, Kim K, Kwon IC, Lee KY. Intracellular glucose-depriving polymer micelles for antiglycolytic cancer treatment. Adv Mater. 2022;16: e2207342.
Qiao T, Xiong Y, Feng Y, Guo W, Zhou Y, Zhao J, et al. Inhibition of LDH-A by oxamate enhances the efficacy of anti-PD-1 treatment in an NSCLC humanized mouse model. Front Oncol. 2021;11: 632364.
Im DK, Cheong H, Lee JS, Oh MK, Yang KM. Protein kinase CK2-dependent aerobic glycolysis-induced lactate dehydrogenase A enhances the migration and invasion of cancer cells. Sci Rep. 2019;9(1):5337.
Wu H, Wang Y, Ying M, Jin C, Li J, Hu X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct Target Ther. 2021;6(1):242.
Kim EY, Chung TW, Han CW, Park SY, Park KH, Jang SB, et al. A novel lactate dehydrogenase inhibitor, 1-(phenylseleno)-4-(trifluoromethyl) benzene, suppresses tumor growth through apoptotic cell death. Sci Rep. 2019;9(1):3969.
Lang N, Wang C, Zhao J, Shi F, Wu T, Cao H. Long non-coding RNA BCYRN1 promotes glycolysis and tumor progression by regulating the miR-149/PKM2 axis in non-small-cell lung cancer. Mol Med Rep. 2020;21(3):1509–16.
Chhipa AS, Patel S. Targeting pyruvate kinase muscle isoform 2 (PKM2) in cancer: what do we know so far? Life Sci. 2021;1(280): 119694.
Shi L, Pan H, Liu Z, Xie J, Han W. Roles of PFKFB3 in cancer. Signal Transduct Target Ther. 2017;2:17044.
Halford S, Veal GJ, Wedge SR, Payne GS, Bacon CM, Sloan P, et al. A phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin Cancer Res. 2023;29(8):1429–39.
Goldberg FW, Kettle JG, Lamont GM, Buttar D, Ting AKT, McGuire TM, et al. Discovery of clinical candidate AZD0095, a selective inhibitor of monocarboxylate transporter 4 (MCT4) for oncology. J Med Chem. 2023;66(1):384–97.
Liberti MV, Locasale JW. The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–8.
Funding
Work presented in this paper was supported by the National Science Centre (Poland) grant number 2018/31/N/NZ5/03561.
Author information
Authors and Affiliations
Contributions
JS—manuscript writing, AP—manuscript writing, JS—manuscript edition and writing, SET—manuscript writing, figure preparation, project administration, BO—creating the concept of the article, manuscript writing and editing, KAKP—creating the concept of the article, manuscript writing and editing, supervision of the team work. All authors have participated in the literature search and article concept discussion. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jaworska, M., Szczudło, J., Pietrzyk, A. et al. The Warburg effect: a score for many instruments in the concert of cancer and cancer niche cells. Pharmacol. Rep 75, 876–890 (2023). https://doi.org/10.1007/s43440-023-00504-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00504-1