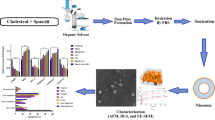
Abstract
Background
Phytochemicals and their derivatives are good options to improve treatment efficiency in cancer patients. Artemisinin (ART) and metformin (MET) are widely used phytochemicals to treat various types of cancers. However, their application because of their dose-dependent side effects, and poor bioavailability brings several challenges. Niosome is a novel nanocarrier that is the best choice to encapsulate both lipophilic and hydrophilic drugs. In this study, we synthesized and characterized various formulations of PEGylated (polyethylene glycol) niosomal nanoparticles co-loaded with ART-MET and evaluated their anticancer effect on A549 lung cancer cells.
Methods
Various formulations of PEGylated noisome were prepared by the thin-film hydration method and characterized in size, morphology, release pattern, and physicochemical structure. The cytotoxic effect of the free ART-MET and optimized PEGylated niosomal nanoparticles loaded with ART-MET on A549 cells were evaluated by MTT assay. Furthermore, the Real-time PCR (RT-PCR) technique used to evaluate apoptotic and anti-apoptotic gene expression.
Results
The size, encapsulation efficiency (EE), and polydispersity index (PDI) of the optimized nanoparticles are 256 nm, 95%, and 0.202, respectively. Additionally, due to the PEGylation hydrophilic character, there is a major consideration of the high impact of PEGylation on reducing niosome size. According to the results of the MTT assay, free ART-MET and ART-MET-loaded niosomal nanoparticles showed dose-dependent toxicity and inhibits the growth of A549 lung cancer cells. Furthermore, the RT-PCR results indicated that ART-MET-loaded niosomal nanoparticles have a higher anti-proliferative effect by inhibiting anti-apoptotic and inducing apoptotic gene expression in A549 lung cancer cells.
Conclusions
Our study revealed that the simultaneous use of ART and MET in the optimized PEGylated niosomal nanoparticles delivery system could be an appropriate approach to improve the effectiveness of lung cancer treatment.
Graphical abstract







Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the supplementary files.
Abbreviations
- AFM:
-
Atomic force microscopy
- ART:
-
Artemisinin
- Chol:
-
Cholesterol
- DLS:
-
Dynamic light scattering
- EE:
-
Encapsulation efficiency
- FBS:
-
Fetal bovine serum
- FE-SEM:
-
Field emission scanning electron microscopes
- MET:
-
Metformin
- Nio:
-
Niosome
- Nio-ART:
-
Niosome loaded artemisinin
- Nio-MET:
-
Niosome loaded metformin
- PBS:
-
Phosphate-buffered saline
- PDI:
-
Polydispersity index
- PEG:
-
Polyethylene glycol
- TEM:
-
Transmission electron microscopy
- TFH:
-
Thin-film hydration
- WHO:
-
World health organization
- ZP:
-
Zeta potential
References
WH Organization. WHO report on cancer: setting priorities, investing wisely and providing care for all. Geneva: World Health Organization; 2020.
Shin H, Oh S, Hong S, Kang M, Kang D, Ji Y-g, et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. 2020;14(5):5435–44.
Petrovic D, Bodinier B, Dagnino S, Whitaker M, Karimi M, Campanella G, et al. Epigenetic mechanisms of lung carcinogenesis involve differentially methylated CpG sites beyond those associated with smoking. Eur J Epidemiol. 2022;37:1–12.
Tran VV, Park D, Lee Y-C. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int J Environ Health Res. 2020;17(8):2927.
Willers H, Stinchcombe TE, Barriger RB, Chetty IJ, Ginsburg ME, Kestin LL, et al. ACR appropriateness criteria® induction and adjuvant therapy for N2 non–small-cell lung cancer. Am J Clin Oncol. 2015;38(2):197–205.
Travis WD. Pathology & genetics tumours of the lung, pleura, thymus and heart. World Health Organization classification of tumours. 2004;10(3):341
Suster DI, Mino-Kenudson M. Molecular pathology of primary non-small cell lung cancer. Arch Med Res. 2020;51(8):784–98.
Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell. 2002;1(1):49–52.
Colby TV, Wistuba II, Gazdar A. Precursors to pulmonary neoplasia. Adv Anat Pathol. 1998;5(4):205–15.
Khazei K, Mohajeri N, Bonabi E, Turk Z, Zarghami N. New insights toward nanostructured drug delivery of plant-derived polyphenol compounds: cancer treatment and gene expression profiles. Curr Cancer Drug Targets. 2021;21(8):689–701.
Kong M, Xie K, Lv M, Li J, Yao J, Yan K, et al. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed Pharmacother. 2021;133: 110975.
Khodadadi M, Jafari-Gharabaghlou D, Zarghami N. An update on mode of action of metformin in modulation of meta-inflammation and inflammaging. Pharmacol Rep. 2022;74:310–22.
Hassani N, Jafari-Gharabaghlou D, Dadashpour M, Zarghami N. The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022;194:1–16.
Ghasemali S, Nejati-Koshki K, Akbarzadeh A, Tafsiri E, Zarghami N, Rahmati-Yamchi M, et al. Inhibitory effects of β-cyclodextrin-helenalin complexes on H-TERT gene expression in the T47D breast cancer cell line-results of real time quantitative PCR. Asian Pac J Cancer Prev. 2013;14(11):6949–53.
Alibakhshi A, Ranjbari J, Pilehvar-Soltanahmadi Y, Nasiri M, Mollazade M, Zarghami N. An update on phytochemicals in molecular target therapy of cancer: potential inhibitory effect on telomerase activity. Curr Med Chem. 2016;23(22):2380–93.
Mohammadian F, Abhari A, Dariushnejad H, Nikanfar A, Pilehvar-Soltanahmadi Y, Zarghami N. Effects of chrysin-PLGA-PEG nanoparticles on proliferation and gene expression of miRNAs in gastric cancer cell line. Iran J Cancer Prev. 2016. https://doi.org/10.17795/ijcp-4190.
Tavakoli F, Jahanban-Esfahlan R, Seidi K, Jabbari M, Behzadi R, Pilehvar-Soltanahmadi Y, et al. Effects of nano-encapsulated curcumin-chrysin on telomerase, MMPs and TIMPs gene expression in mouse B16F10 melanoma tumour model. Artif Cells Nanomed Biotechnol. 2018;46(sup2):75–86.
Slezáková S, Ruda-Kucerova J. Anticancer activity of artemisinin and its derivatives. Anticancer res. 2017;37(11):5995–6003.
Jafari-Gharabaghlou D, Pilehvar-Soltanahmadi Y, Dadashpour M, Mota A, Vafajouy-Jamshidi S, Faramarzi L, et al. Combination of metformin and phenformin synergistically inhibits proliferation and hTERT expression in human breast cancer cells. Iran J Basic Med Sci. 2018;21(11):1167.
Salmani Javan E, Lotfi F, Jafari-Gharabaghlou D, Mousazadeh H, Dadashpour M, Zarghami N. Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian Pac J Cancer Prev. 2022;23(2):519–27.
Alagheband Y, Jafari-gharabaghlou D, Imani M, Mousazadeh H, Dadashpour M, Firouzi-Amandi A, et al. Design and fabrication of a dual-drug loaded nano-platform for synergistic anticancer and cytotoxicity effects on the expression of leptin in lung cancer treatment. J Drug Deliv Sci Technol. 2022;73: 103389.
Javidfar S, Pilehvar-Soltanahmadi Y, Farajzadeh R, Lotfi-Attari J, Shafiei-Irannejad V, Hashemi M, et al. The inhibitory effects of nano-encapsulated metformin on growth and hTERT expression in breast cancer cells. J Drug Deliv Sci Technol. 2018;43:19–26.
Wahdan-Alaswad RS, Thor AD. Metformin activity against breast cancer: mechanistic differences by molecular subtype and metabolic conditions. London: IntechOpen; 2020.
Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8(1):88–96.
Milewska S, Niemirowicz-Laskowska K, Siemiaszko G, Nowicki P, Wilczewska AZ, Car H. Current trends and challenges in pharmacoeconomic aspects of nanocarriers as drug delivery systems for cancer treatment. Int J Nanomed. 2021;16:6593.
Zamani R, Aval SF, Pilehvar-Soltanahmadi Y, Nejati-Koshki K, Zarghami N. Recent advances in cell electrospining of natural and synthetic nanofibers for regenerative medicine. Drug res. 2018;68(08):425–35.
Khan MI, Hossain MI, Hossain MK, Rubel M, Hossain K, Mahfuz A, et al. Recent progress in nanostructured smart drug delivery systems for cancer therapy: a review. ACS Appl Bio Mater. 2022;5(3):971–1012.
Akbarzadeh I, Keramati M, Azadi A, Afzali E, Shahbazi R, Norouzian D, et al. Optimization, physicochemical characterization, and antimicrobial activity of a novel simvastatin nano-niosomal gel against E. coli and S. aureus. Chem Phys Lipids. 2021;234:105019.
Akbarzadeh I, Saremi Poor A, Yaghmaei S, Norouzian D, Noorbazargan H, Saffar S, et al. Niosomal delivery of simvastatin to MDA-MB-231 cancer cells. Drug Dev Ind Pharm. 2020;46(9):1535–49.
Manosroi A, Khanrin P, Lohcharoenkal W, Werner RG, Götz F, Manosroi W, et al. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm. 2010;392(1–2):304–10.
Mohawed OA, El-Ashmoony M, Elgazayerly ON. Niosome-encapsulated clomipramine for transdermal controlled delivery. Int J Pharm Pharm Sci. 2014;6(9):567–75.
Usama A, Fetih G, El-Faham T. Performance of meloxicam niosomal gel formulations for transdermal drug delivery. Br J Pharm Res. 2016;12(2):1–14.
Azeem A, Ahmad FJ, Khan ZI, Talegaonkar S. Nonionic surfactant vesicles as a carrier for transdermal delivery of frusemide. J Dispers Sci Technol. 2008;29(5):723–30.
Abdelkader H, Wu Z, Al-Kassas R, Alany RG. Niosomes and discomes for ocular delivery of naltrexone hydrochloride: morphological, rheological, spreading properties and photo-protective effects. Int J Pharm. 2012;433(1–2):142–8.
Zeng W, Li Q, Wan T, Liu C, Pan W, Wu Z, et al. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Coll Surf B Biointerfaces. 2016;141:28–35.
Bansal S, Aggarwal G, Chandel P, Harikumar S. Design and development of cefdinir niosomes for oral delivery. J Pharm Bioallied Sci. 2013;5(4):318.
Bini K, Akhilesh D, Prabhakara P, Kamath J. Development and characterization of non-ionic surfactant vesicles (niosomes) for oral delivery of lornoxicam. Int J Drug Dev Res. 2012;4(3):147–54.
Marianecci C, Paolino D, Celia C, Fresta M, Carafa M, Alhaique F. Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: characterization and interaction with human lung fibroblasts. J Control Release. 2010;147(1):127–35.
De A, Venkatesh N, Senthil M, Sanapalli BKR, Shanmugham R, Karri VVSR. Smart niosomes of temozolomide for enhancement of brain targeting. Nanobiomedicine. 2018;5:1849543518805355.
Abdelkader H, Alani AW, Alany RG. Recent advances in non-ionic surfactant vesicles (niosomes): self-assembly, fabrication, characterization, drug delivery applications and limitations. Drug deliv. 2014;21(2):87–100.
Azmin M, Florence A, Handjani-Vila R, Stuart J, Vanlerberghe G, Whittaker J. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J Pharm Pharmacol. 1985;37(4):237–42.
Jain C, Vyas S, Dixit V. Niosomal system for delivery of rifampicin to lymphatics. Indian J Pharm Sci. 2006;68(5):575.
Ansari MJ, Anwer MK, Jamil S, Al-Shdefat R, Ali BE, Ahmad MM, et al. Enhanced oral bioavailability of insulin-loaded solid lipid nanoparticles: pharmacokinetic bioavailability of insulin-loaded solid lipid nanoparticles in diabetic rats. Drug Deliv. 2016;23(6):1972–9.
Ghafelehbashi R, Akbarzadeh I, Yaraki MT, Lajevardi A, Fatemizadeh M, Saremi LH. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int J Pharm. 2019;569: 118580.
Nasseri B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int J Pharm. 2005;300(1–2):95–101.
Mehta SK, Jindal N, Kaur G. Quantitative investigation, stability and in vitro release studies of anti-TB drugs in triton niosomes. Coll Surf B Biointerfaces. 2011;87(1):173–9.
Shafiei-Irannejad V, Samadi N, Yousefi B, Salehi R, Velaei K, Zarghami N. Metformin enhances doxorubicin sensitivity via inhibition of doxorubicin efflux in P-gp-overexpressing MCF-7 cells. Chem Biol Drug Des. 2018;91(1):269–76.
Eatemadi A, Daraee H, Aiyelabegan HT, Negahdari B, Rajeian B, Zarghami N. Synthesis and characterization of chrysin-loaded PCL-PEG-PCL nanoparticle and its effect on breast cancer cell line. Biomed Pharmacother. 2016;84:1915–22.
Sadeghzadeh H, Pilehvar-Soltanahmadi Y, Akbarzadeh A, Dariushnejad H, Sanjarian F, Zarghami N. The effects of nanoencapsulated curcumin-Fe3O4 on proliferation and hTERT gene expression in lung cancer cells. Anti-Cancer Agents Med Chem. 2017;17(10):1363–73.
Muzzalupo R, Tavano L. Niosomal drug delivery for transdermal targeting: recent advances. Res Rep Transdermal Drug Deliv. 2015;4:23–33.
Rochdy Haj-Ahmad R, Ali Elkordy A, Shu CC. In vitro characterisation of span™ 65 niosomal formulations containing proteins. Curr Drug Deliv. 2015;12(5):628–39.
Abdelkader H, Farghaly U, Moharram H. Effects of surfactant type and cholesterol level on niosomes physical properties and in vivo ocular performance using timolol maleate as a model drug. J Pharm Investig. 2014;44(5):329–37.
Abdelkader H, Ismail S, Kamal A, Alany RG. Design and evaluation of controlled-release niosomes and discomes for naltrexone hydrochloride ocular delivery. J Pharm Sci. 2011;100(5):1833–46.
Li Q, Li Z, Zeng W, Ge S, Lu H, Wu C, et al. Proniosome-derived niosomes for tacrolimus topical ocular delivery: in vitro cornea permeation, ocular irritation, and in vivo anti-allograft rejection. Eur J Pharm Sci. 2014;62:115–23.
Basiri L, Rajabzadeh G, Bostan A. α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food chem. 2017;221:620–8.
Shaker DS, Shaker MA, Hanafy MS. Cellular uptake, cytotoxicity and in-vivo evaluation of tamoxifen citrate loaded niosomes. Int J Pharm. 2015;493(1–2):285–94.
Kassem MA, El-Sawy HS, Abd-Allah FI, Abdelghany TM, Khalid M. Maximizing the therapeutic efficacy of imatinib mesylate–loaded niosomes on human colon adenocarcinoma using Box-Behnken design. J Pharm Sci. 2017;106(1):111–22.
Patel J, Ketkar S, Patil S, Fearnley J, Mahadik KR, Paradkar AR. Potentiating antimicrobial efficacy of propolis through niosomal-based system for administration. Integr Med Res. 2015;4(2):94–101.
Bragagni M, Mennini N, Furlanetto S, Orlandini S, Ghelardini C, Mura P. Development and characterization of functionalized niosomes for brain targeting of dynorphin-B. Eur J Pharm Biopharm. 2014;87(1):73–9.
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;185:22–36.
Husseini GA, De La Rosa MAD, Richardson ES, Christensen DA, Pitt WG. The role of cavitation in acoustically activated drug delivery. J Control Release. 2005;107(2):253–61.
Varshosaz J, Pardakhty A, Hajhashemi V-i, Najafabadi AR. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003;10(4):251–62.
Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, et al. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010;27(7):618–27.
Marwa A, Omaima S, Hanaa E-G, Mohammed A-S. Preparation and in-vitro evaluation of diclofenac sodium niosomal formulations. Int J Pharm Sci Res. 2013;4(5):1757.
Richardson ES, Pitt WG, Woodbury DJ. The role of cavitation in liposome formation. Biophys J. 2007;93(12):4100–7.
Lee S-C, Lee K-E, Kim J-J, Lim S-H. The effect of cholesterol in the liposome bilayer on the stabilization of incorporated retinol. J Liposome Res. 2005;15(3–4):157–66.
Cable C. An examination of the effect of surface modifications on the physicochemical and biological properties of non-ionic surfactant vesicles (surfactant vesicles, physicochemical properties), in Ph. D. Thesis 1989. Strathclyde: Glasgow
Patel H. Liposomes: a practical approach: edited by RRC New. Oxford: Oxford University Press; 1990. p. 301 (£ 22.50. No longer published by Elsevier; 1990).
Gupta M, Vaidya B, Mishra N, Vyas SP. Effect of surfactants on the characteristics of fluconazole niosomes for enhanced cutaneous delivery. Artif Cells Blood Substit Immobil Biotechnol. 2011;39(6):376–84.
Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70.
Khazaeli P, Pardakhty A, Shoorabi H. Caffeine-loaded niosomes: characterization and in vitro release studies. Drug deliv. 2007;14(7):447–52.
Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int J Pharm. 1994;105:1.
Sadeghi S, Bakhshandeh H, Cohan RA, Peirovi A, Ehsani P, Norouzian D. Synergistic anti-staphylococcal activity of niosomal recombinant lysostaphin-LL-37. Int J Nanomed. 2019;14:9777.
Barani M, Mirzaei M, Torkzadeh-Mahani M, Nematollahi MH. Lawsone-loaded niosome and its antitumor activity in MCF-7 breast cancer cell line: a nano-herbal treatment for cancer. DARU J Pharm Sci. 2018;26(1):11–7.
Hajizadeh MR, Maleki H, Barani M, Fahmidehkar MA, Mahmoodi M, Torkzadeh-Mahani M. In vitro cytotoxicity assay of D-limonene niosomes: an efficient nano-carrier for enhancing solubility of plant-extracted agents. Res Pharm Sci. 2019;14(5):448.
Hao Y, Zhao F, Li N, Yang Y. Studies on a high encapsulation of colchicine by a niosome system. Int J Pharm. 2002;244(1–2):73–80.
Pardakhty A, Moazeni E, Varshosaz J, Hajhashemi V, Najafabadi AR. Pharmacokinetic study of niosome-loaded insulin in diabetic rats. DARU J Pharm Sci. 2011;19(6):404.
Akbarzadeh I, Yaraki MT, Bourbour M, Noorbazargan H, Lajevardi A, Shilsar SMS, et al. Optimized doxycycline-loaded niosomal formulation for treatment of infection-associated prostate cancer: an in-vitro investigation. J Drug Deliv Sci Technol. 2020;57: 101715.
Seras-Cansell M, Ollivon M, Lesieur S. Generation of non-ionic monoalkyl amphiphile-cholesterol vesicles: evidence of membrane impermeability to octyl glucoside. STP Pharma Sci. 1996;6(1):12–20.
Yoon O, Roh J. Downregulation of KLF4 and the Bcl-2/Bax ratio in advanced epithelial ovarian cancer. Oncol Lett. 2012;4(5):1033–6.
Chen J, Zhang L, Hao M. Effect of artemisinin on proliferation and apoptosis-related protein expression in vivo and in vitro. Saudi J Biol Sci. 2018;25(7):1488–93.
Acknowledgements
We gratefully acknowledge the Tabriz University of Medical Sciences and Islamic Azad University Science and Research Branch of Tehran for all supporting and laboratory facilities for this study.
Funding
We thank the Tabriz University of Medical Sciences for all supporting.
Author information
Authors and Affiliations
Contributions
RS: investigation, validation, methodology, data curation, writing—original draft. DJ-G: supervision, validation, writing—review and editing. ZM and HS: writing—review and editing. NZ: supervision, validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
43440_2023_462_MOESM1_ESM.docx
Supplementary file1 Table S1. Optimizing PEGylated-ART-MET-loaded niosomes by D-optimal design used by design expert 12.0.3.0 software. N: noisome, ART: artemisinin, MET: metformin, S: Surfactant type, A: The amount of surfactant (mM), B: The amount of cholesterol (mM), P: The amount of polyethylene glycol (mM), PDI: polydispersity index, EE: encapsulation efficiency (%). Results are presented as mean ± standard deviation; in all niosomal formulations, entrapment efficiency was significantly different from each other p <0.05 (DOCX 13 KB)
43440_2023_462_MOESM2_ESM.docx
Supplementary file2 Table S2. Various formulations of niosomal NPs. F: formulation. S: The amount of surfactant, Ch: The amount of cholesterol, HLB: hydrophilic-lipophilic balance, S/Ch: molar ratio of surfactant to cholesterol. Experiments were carried out three times and the results are presented as mean ± SD (n = 3) (DOCX 12 KB)
43440_2023_462_MOESM3_ESM.docx
Supplementary file3 Table S3. ART-MET-loaded niosomal NPs stability and changes in particle size (nm), entrapment efficiency (EE), and polydispersity index (PDI) at 4 ± 0.5 °c and 25 ± 1 °C during two months. Size (nm), PDI: polydispersity index, EE: entrapment efficiency (DOCX 12 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shahbazi, R., Jafari-Gharabaghlou, D., Mirjafary, Z. et al. Design and optimization various formulations of PEGylated niosomal nanoparticles loaded with phytochemical agents: potential anti-cancer effects against human lung cancer cells. Pharmacol. Rep 75, 442–455 (2023). https://doi.org/10.1007/s43440-023-00462-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-023-00462-8