Abstract
Background
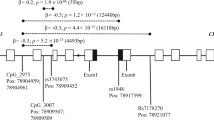
Past studies have established the association of CHRNA5-A3-B4 gene cluster variants with various smoking behaviors in different ethnicities, yet no such study has been reported in Bengali ethnicity to date.
Methods
A case–control study with 129 smokers and 111 non-smokers was conducted and genotyped using polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method aimed to manifest the association of three SNPs in this gene cluster with smoking status (SS) in a Bangladeshi population.
Results
The non-synonymous CHRNA5 rs1s6969968 and 3′-UTR variant CHRNA3 rs578776 polymorphisms were found to have a strong association with SS. Carriers of polymorphic ‘A’ allele of rs16969968 showed 1.51-fold more risk of being smokers (adjusted OR = 1.51, 95% CI 0.88–2.57, p = 0.128); whereas, rs578776 polymorphic ‘A’ allele carriers showed 0.595-fold less risk of being smokers (adjusted OR = 1.51, 95% CI 0.88–2.57, p = 0.006). Comparing smokers and non-smokers, A/A mutant homozygous genotypes of rs578776 and rs16969968 variants pose 0.369-fold (95% CI 0.177–0.77, p = 0.008) and 3.3-fold (95% CI 0.66–16.46, p = 0.14) more risk for positive SS, respectively. No genotypic association for SS was found with intronic variant CHRNB4 rs11072768 (T/G; adjusted OR = 0.827, 95% CI 0.457–1.499, p = 0.532 and G/G; adjusted OR = 0.992, 95% CI 0.455–2.167, p = 0.985). Combination of rs16969968-positive/rs578776-negative polymorphic variants possesses the risk of positive SS in young adults. Furthermore, two new haplotypes (AAT and AAG) were identified in Bangladeshi population and GAG (OR = 0.45, 95% CI 0.25–0.8, p = 0.006) haplotype was found to be a protective factor for SS.
Conclusion
Nicotinic acetylcholine gene cluster CHRNA5-A3-B4 variants rs16969968 and rs578776 are associated with SS in a Bangladeshi population. Large-scale studies are warranted to establish this genotype–phenotype correlation.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
WHO EMRO. Number of males using tobacco globally on the decline, showing that government-led control efforts work to save lives, protect health, beat tobacco | News | Media centre n.d. http://www.emro.who.int/media/news/number-of-males-using-tobacco-globally-on-the-decline-showing-that-government-led-control-efforts-work-to-save-lives-protect-health-beat-tobacco.html (Accessed July 3, 2020).
Hasan M. WHO: tobacco responsible for 1 in 5 deaths in Bangladesh. Dhaka Tribune 2018. https://www.dhakatribune.com/health/2018/06/01/tobacco-1-in-5-deaths-bangladesh (Accessed July 3, 2020).
Faruque G, Wadood S, Ahmed M, Parven R, Huq I, Chowdhury S (2019). The economic cost of tobacco use in Bangladesh: a health cost approach. Bangladesh Cancer SOciety.
Edwards KL, Austin MA, Jarvik GP. Evidence for genetic influences on smoking in adult women twins. Clin Genet. 1995;47:236–44. https://doi.org/10.1111/j.1399-0004.1995.tb04303.x.
Facchini FS, Hollenbeck CB, Jeppesen J, Chen YD, Reaven GM. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–30. https://doi.org/10.1016/0140-6736(92)90730-q.
Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2007. https://doi.org/10.1002/14651858.CD000031.pub3.
Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004. https://doi.org/10.1002/14651858.CD000146.pub2.
Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, et al. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102:413–22. https://doi.org/10.1111/j.1360-0443.2006.01694.x.
Lessov CN, Martin NG, Statham DJ, Todorov AA, Slutske WS, Bucholz KK, et al. Defining nicotine dependence for genetic research: evidence from Australian twins. Psychol Med. 2004;34:865–79. https://doi.org/10.1017/s0033291703001582.
Munafò MR, Johnstone EC. Genes and cigarette smoking. Addiction. 2008;103:893–904. https://doi.org/10.1111/j.1360-0443.2007.02071.x.
Arinami T, Ishiguro H, Onaivi ES. Polymorphisms in genes involved in neurotransmission in relation to smoking. Eur J Pharmacol. 2000;410:215–26. https://doi.org/10.1016/s0014-2999(00)00816-5.
Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking–a study of male twins. N Engl J Med. 1992;327:829–33. https://doi.org/10.1056/NEJM199209173271201.
True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, et al. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–87.
Heath AC, Kirk KM, Meyer JM, Martin NG. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behav Genet. 1999;29:395–407. https://doi.org/10.1023/A:1021670703806.
Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29:299–308. https://doi.org/10.1017/s0033291798008022.
Madden PAF, Heath AC, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The genetics of smoking persistence in men and women: a multicultural study. Behav Genet. 1999;29:423–31. https://doi.org/10.1023/A:1021674804714.
Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999. https://doi.org/10.1080/14622299050011811 (1 Suppl 2:S51-57; discussion S69-70).
Brunzell DH, Stafford AM, Dixon CI. Nicotinic receptor contributions to smoking: insights from human studies and animal models. Curr Addict Rep. 2015;2:33–46. https://doi.org/10.1007/s40429-015-0042-2.
Cosgrove KP, Esterlis I, Sandiego C, Petrulli R, Morris ED. Imaging tobacco smoking with PET and SPECT. Curr Top Behav Neurosci. 2015;24:1–17. https://doi.org/10.1007/978-3-319-13482-6_1.
Dani JA. Chapter one: neuronal nicotinic acetylcholine receptor structure and function and response to nicotine. In: De Biasi M, editor. International review of neurobiology, vol. 124. United States: Academic press; 2015. p. 3–19.
Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–40. https://doi.org/10.1038/ng.572.
Mohamed TS, Jayakar SS, Hamouda AK. Orthosteric and allosteric ligands of nicotinic acetylcholine receptors for smoking cessation. Front Mol Neurosci. 2015;8:71. https://doi.org/10.3389/fnmol.2015.00071.
Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet. 2010;42:448–53. https://doi.org/10.1038/ng.573.
Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–7. https://doi.org/10.1038/ng.571.
Wang Q, Li S, Pan L, Li H, Yang X, Jiang F, et al. Association between variants in nicotinic acetylcholine receptor genes and smoking cessation in a Chinese rural population. Am J Addict. 2016;25:297–300. https://doi.org/10.1111/ajad.12383.
Perry DC, Dávila-García MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–52.
Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–30. https://doi.org/10.1038/nrcardio.2013.8.
Wen L, Jiang K, Yuan W, Cui W, Li MD. Contribution of variants in CHRNA5/A3/B4 gene cluster on chromosome 15 to tobacco smoking: from genetic association to mechanism. Mol Neurobiol. 2016;53:472–84. https://doi.org/10.1007/s12035-014-8997-x.
Gotti C, MichaelJ M, Millar NS, Wonnacott S. Nicotinic acetylcholine receptors (version 2019.4) in the IUPHAR/BPS guide to pharmacology database. GtoPdb CITE. 2019. https://doi.org/10.2218/gtopdb/F76/2019.4.
Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves. Ann NY Acad Sci. 2003;998:41–52. https://doi.org/10.1196/annals.1254.007.
Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–22. https://doi.org/10.1038/ng.109.
Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M. A CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology. 2015;40:2813–21. https://doi.org/10.1038/npp.2015.131.
Pintarelli G, Galvan A, Pozzi P, Noci S, Pasetti G, Sala F, et al. Pharmacogenetic study of seven polymorphisms in three nicotinic acetylcholine receptor subunits in smoking-cessation therapies. Sci Rep. 2017;7:16730. https://doi.org/10.1038/s41598-017-16946-6.
Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–42. https://doi.org/10.1038/nature06846.
Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–71. https://doi.org/10.1176/appi.ajp.2008.07111711.
Hong LE, Yang X, Wonodi I, Hodgkinson CA, Goldman D, Stine OC, et al. A CHRNA5 allele related to nicotine addiction and schizophrenia. Genes Brain Behav. 2011;10:530–5. https://doi.org/10.1111/j.1601-183X.2011.00689.x.
Sherva R, Wilhelmsen K, Pomerleau CS, Chasse SA, Rice JP, Snedecor SM, et al. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction. 2008;103:1544–52. https://doi.org/10.1111/j.1360-0443.2008.02279.x.
Kendler KS, Chen X, Dick D, Maes H, Gillespie N, Neale MC, et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders. Nat Neurosci. 2012;15:181–9. https://doi.org/10.1038/nn.3018.
Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, et al. The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res. 2009;69:6848–56. https://doi.org/10.1158/0008-5472.CAN-09-0786.
Zhu AZX, Renner CC, Hatsukami DK, Benowitz NL, Tyndale RF. CHRNA5-A3-B4 genetic variants alter nicotine intake and interact with tobacco use to influence body weight in Alaska Native tobacco users. Addiction. 2013;108:1818–28. https://doi.org/10.1111/add.12250.
Baker TB, Weiss RB, Bolt D, von Niederhausern A, Fiore MC, Dunn DM, et al. Human neuronal acetylcholine receptor A5–A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res. 2009;11:785–96. https://doi.org/10.1093/ntr/ntp064.
Hamidovic A, Kasberger JL, Young TR, Goodloe RJ, Redline S, Buxbaum SG, et al. Genetic variability of smoking persistence in African Americans. Cancer Prev Res (Phila). 2011;4:729–34. https://doi.org/10.1158/1940-6207.CAPR-10-0362.
Li MD, Yoon D, Lee J-Y, Han B-G, Niu T, Payne TJ, et al. Associations of variants in CHRNA5/A3/B4 gene cluster with smoking behaviors in a Korean population. PLoS ONE. 2010;5:e12183. https://doi.org/10.1371/journal.pone.0012183.
Zhang Y, Jiang M, Li Q, Liang W, He Q, Chen W, et al. Chromosome 15q25 (CHRNA3-CHRNB4) variation indirectly impacts lung cancer risk in Chinese males. PLoS ONE. 2016;11:e0149946. https://doi.org/10.1371/journal.pone.0149946.
Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, et al. A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet. 2008;4:e1000125. https://doi.org/10.1371/journal.pgen.1000125.
World Health Organization, editor (1998). Guidelines for controlling and monitoring the tobacco epidemic. Geneva: World Health Organization.
World medical association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. https://doi.org/10.1001/jama.2013.281053.
Bin Sayeed MS, Hasan Apu MN, Munir MT, Ahmed MU, Islam MS, Haq MM, et al. Prevalence of CYP2C19 alleles, pharmacokinetic and pharmacodynamic variation of clopidogrel and prasugrel in Bangladeshi population. Clin Exp Pharmacol Physiol. 2015;42:451–7. https://doi.org/10.1111/1440-1681.12390.
Auton A, Abecasis GR, Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. https://doi.org/10.1038/nature15393.
Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet. 2009;18:2922–7. https://doi.org/10.1093/hmg/ddp216.
Grucza RA, Johnson EO, Krueger RF, Breslau N, Saccone NL, Chen L-S, et al. Incorporating age at onset of smoking into genetic models for nicotine dependence: evidence for interaction with multiple genes. Addict Biol. 2010;15:346–57. https://doi.org/10.1111/j.1369-1600.2010.00220.x.
Lassi G, Tan V, Mahedy L, Oliveira ASF, Dyer ML, Drax K, et al. Exploration of the role of CHRNA5-A3-B4 genotype in smoking behaviours. BioRxiv. 2019. https://doi.org/10.1101/818252.
Li MD. Involvement of Variants in gene clusters CHRNA5/A3/B4 on chromosome 15 to smoking behaviors and lung cancer. In: Li MD, editor. Tobacco smoking addiction: epidemiology, genetics, mechanisms, and treatment. Singapore: Springer; 2018. p. 47–69.
Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:275–84. https://doi.org/10.1002/ajmg.b.31155.
Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–46. https://doi.org/10.1016/j.biopsych.2007.10.024.
Sorice R, Bione S, Sansanelli S, Ulivi S, Athanasakis E, Lanzara C, et al. Association of a variant in the CHRNA5-A3-B4 gene cluster region to heavy smoking in the Italian population. Eur J Hum Genet. 2011;19:593–6. https://doi.org/10.1038/ejhg.2010.240.
Adjangba C, Border R, Villela PNR, Ehringer MA, Evans LM. Little evidence of modified genetic effect of rs16969968 on heavy smoking based on age of onset of smoking. MedRxiv. 2020. https://doi.org/10.1101/2020.04.22.20071407 (2020.04.22.20071407).
Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, et al. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–73. https://doi.org/10.1038/sj.mp.4002154.
Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, et al. Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS ONE. 2009;4:e4653. https://doi.org/10.1371/journal.pone.0004653.
Leung T, Bergen A, Munafò MR, De Ruyck K, Selby P, De Luca V. Effect of the rs1051730–rs16969968 variant and smoking cessation treatment: a meta-analysis. Pharmacogenomics. 2015;16:713–20. https://doi.org/10.2217/pgs.15.34.
Lips EH, Gaborieau V, McKay JD, Chabrier A, Hung RJ, Boffetta P, et al. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39:563–77. https://doi.org/10.1093/ije/dyp288.
Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Park JY, Drobes DJ, et al. Variation in the α 5 nicotinic acetylcholine receptor subunit gene predicts cigarette smoking intensity as a function of nicotine content. Pharmacogenomics J. 2014;14:70–6. https://doi.org/10.1038/tpj.2012.50.
Pandey N, Pal S, Sharma LK, Guleria R, Mohan A, Srivastava T. SNP rs16969968 as a strong predictor of nicotine dependence and lung cancer risk in a north indian population. Asian Pac J Cancer Prev. 2017;18:3073–9. https://doi.org/10.22034/APJCP.2017.18.11.3073.
Pérez-Morales R, González-Zamora A, González-Delgado MF, Rincón EYC, Calderón EHO, Martínez-Ramírez OC, et al. CHRNA3 rs1051730 and CHRNA5 rs16969968 polymorphisms are associated with heavy smoking, lung cancer, and chronic obstructive pulmonary disease in a mexican population. Ann Hum Genet. 2018;82:415–24. https://doi.org/10.1111/ahg.12264.
Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–51. https://doi.org/10.1038/380347a0.
Sciaccaluga M, Moriconi C, Martinello K, Catalano M, Bermudez I, Stitzel JA, et al. Crucial role of nicotinic α5 subunit variants for Ca2+ fluxes in ventral midbrain neurons. FASEB J. 2015;29:3389–98. https://doi.org/10.1096/fj.14-268102.
Stevens VL, Bierut LJ, Talbot JT, Wang JC, Sun J, Hinrichs AL, et al. Nicotinic receptor gene variants influence susceptibility to heavy smoking. Cancer Epidemiol Biomarkers Prev. 2008;17:3517–25. https://doi.org/10.1158/1055-9965.EPI-08-0585.
Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–76. https://doi.org/10.1124/mol.106.030445.
Nees F, Witt SH, Lourdusamy A, Vollstädt-Klein S, Steiner S, Poustka L, et al. Genetic risk for nicotine dependence in the cholinergic system and activation of the brain reward system in healthy adolescents. Neuropsychopharmacology. 2013;38:2081–9. https://doi.org/10.1038/npp.2013.131.
Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. https://doi.org/10.1093/hmg/ddl438.
Saccone NL, Culverhouse RC, Schwantes-An T-H, Cannon DS, Chen X, Cichon S, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010. https://doi.org/10.1371/journal.pgen.1001053.
Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, et al. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–66. https://doi.org/10.1002/ajmg.b.30828.
Gold AB, Lerman C. Pharmacogenetics of smoking cessation: role of nicotine target and metabolism genes. Hum Genet. 2012. https://doi.org/10.1007/s00439-012-1143-9.
Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol Pharmacol. 2011;79:119–25. https://doi.org/10.1124/mol.110.066357.
Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B, et al. A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA. 2010;107:13509–14. https://doi.org/10.1073/pnas.1004745107.
Acknowledgements
The authors are thankful to the volunteers, their families, nurses and physicians who helped us to the successful completion of the study. The authors would also like to thank the Department of Clinical Pharmacy and Pharmacology, University of Dhaka, Bangladesh to provide lab facilities and other opportunities to carry out the research work.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
N.I.C.: performed all the experiments and drafted the manuscript; T.N.S., M.M.H: assisted in experiments, revised manuscript; I.I.S.: assisted in sample acquisition; N.A.N., S.I.: critically revised manuscript; M.N.H.A.: conceptualized and conceived research design, performed statistical analyses, revised the manuscript, principle investigator.
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no competing interests.
Ethical standards
The current study was in accordance with the Declaration of Helsinki and its further amendments and the ethical committee of the participating institution approved the study protocol.
Informed consent
Each case and control subject signed an informed consent document after they were informed of the study objectives.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chaity, N.I., Sultana, T.N., Hasan, M.M. et al. Nicotinic acetylcholine gene cluster CHRNA5-A3-B4 variants influence smoking status in a Bangladeshi population. Pharmacol. Rep 73, 574–582 (2021). https://doi.org/10.1007/s43440-021-00243-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00243-1