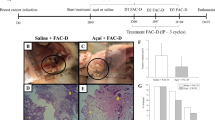
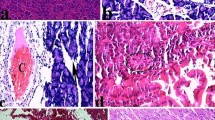
Abstract
Tamoxifen (TAM) is a commonly used drug for breast cancer treatment. Although effective, TAM has deleterious effects on many organs. The toxic effects of TAM on the pancreas and the underlying mechanisms however, have not fully investigated. In the present study, we investigated the effects of TAM on the pancreatic tissue in female rats. We also examined whether cardamom aqueous extract (CAE) protects against TAM-induced pancreatic injury. TAM-intoxicated rats were injected with 45 mg/kg of TAM for 10 days, whereas rats in the CAE-treated group were administered 10 mL/kg of CAE for 20 days, starting 10 days prior to TAM administration. Treatment with TAM resulted in severe degeneration of the pancreatic acini and marked increases in the serum levels of pancreatic lipase, α-amylase, glucose, fatty acids and triglycerides along with decreased insulin serum levels. TAM led to oxidative stress as evident from a significant increase in the pancreatic levels of lipid peroxides and nitric oxide along with the depletion of reduced glutathione, glutathione peroxidase, and superoxide dismutase. Moreover, inflammation was indicated by a significant increase in tumor necrosis factor–α and interleukin-6 levels, enhanced expression of the macrophage recruitment marker; CD68 as well as up-regulated protein levels of toll-like receptor 4 and nuclear factor kappa B and increased p-p38/MAPK ratio; which are important signals in the production of inflammatory cytokines. TAM also markedly increased the pancreatic levels of caspase-3 and BAX reflecting its apoptotic effects. The CAE treatment ameliorated all the biochemical and histological changes induced by TAM. The present study revealed, for the first time, that TAM has toxic effects on the pancreatic tissue through oxidative stress, inflammation and apoptotic effects. The present study also provides evidence that CAE exerts cytoprotective effects against these deleterious effects induced by TAM in the pancreatic tissue.









Similar content being viewed by others
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Lumachi F, Santeufemia DA, Basso SM (2015) Current medical treatment of estrogen receptor-positive breast cancer. World J Biol Chem 6:231–239. https://doi.org/10.4331/wjbc.v6.i3.231
Singh HK, Prasad MS, Kandasamy AK, Dharanipragada K (2016) Tamoxifen-induced hypertriglyceridemia causing acute pancreatitis. J Pharmacol Pharmacother 7:38–40. https://doi.org/10.4103/0976-500X.179365
Xiong R, Zhao J, Gutgesell LM, Wang Y, Lee S, Karumudi B, Zhao H, Lu Y, Tonetti DA, Thatcher GR (2017) Novel selective estrogen receptor downregulators (SERDs) developed against treatment-resistant breast cancer. J Med Chem 60:1325–1342. https://doi.org/10.1021/acs.jmedchem.6b01355
Yang G, Nowsheen S, Aziz K, Georgakilas AG (2013) Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol Ther 139:392–404. https://doi.org/10.1016/j.pharmthera.2013.05.005
Sakhri J, Ben Salem C, Harbi H, Fathallah N, Ltaief R (2010) Severe acute pancreatitis due to tamoxifen-induced hypertriglyceridemia with positive rechallenge. JOP 11:382–384
Ahn SH, Granger A, Rankin MM, Lam CJ, Cox AR, Kushner JA (2019) Tamoxifen suppresses pancreatic β-cell proliferation in mice. PLoS One 14:e0214829. https://doi.org/10.1371/journal.pone.0214829
Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL (2008) Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysis. JAMA 300:2754–2764. https://doi.org/10.1001/jama.2008.824
Owumi E, S, K Olusola J, O Arunsi U, K Oyelere A, (2021) Chlorogenic acid abates oxido-inflammatory and apoptotic responses in the liver and kidney of Tamoxifen-treated rats. Toxicol Res (Camb) 10:345–353. https://doi.org/10.1093/toxres/tfab002
El-Dessouki AM, El Fattah MA, Awad AS, Zaki HF (2018) Zafirlukast and vincamine ameliorate tamoxifen-induced oxidative stress and inflammation: Role of the JNK/ERK pathway. Life Sci 202:78–88. https://doi.org/10.1016/j.lfs.2018.04.002
Parvez S, Tabassum H, Banerjee BD, Raisuddin S (2008) Taurine prevents tamoxifen-induced mitochondrial oxidative damage in mice. Basic Clin Pharmacol Toxicol 102:382–387. https://doi.org/10.1111/j.1742-7843.2008.00208.x
Nazarewicz RR, Zenebe WJ, Parihar A, Larson SK, Alidema E, Choi J, Ghafourifar P (2007) Tamoxifen induces oxidative stress and mitochondrial apoptosis via stimulating mitochondrial nitric oxide synthase. Cancer Res 67:1282–1290. https://doi.org/10.1158/0008-5472.CAN-06-3099
Ribeiro MP, Santos AE, Custódio JB (2014) Mitochondria: the gateway for tamoxifen-induced liver injury. Toxicology 323:10–18. https://doi.org/10.1016/j.tox.2014.05.009
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288:481–487. https://doi.org/10.1016/0003-9861(91)90224-7
Zhou WB, Zhang XX, Cai Y, Sun W, Li H (2019) Osthole prevents tamoxifen-induced liver injury in mice. Acta Pharmacol Sin 40:608–619. https://doi.org/10.1038/s41401-018-0171-y
Rahman MM, Alam MN, Ulla A, Sumi FA, Subhan N, Khan T, Sikder B, Hossain H, Reza HM, Alam MA (2017) Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Dis 16:151. https://doi.org/10.1186/s12944-017-0539-x
Goyal SN, Sharma C, Mahajan UB, Patil CR, Agrawal YO, Kumari S, Arya DS, Ojha S (2015) Protective Effects of Cardamom in Isoproterenol-Induced Myocardial Infarction in Rats. Int J Mol Sci 16:27457–27469. https://doi.org/10.3390/ijms161126040
Akrayi H (2012) Antibacterial Effect of Seed Extracts of Cardamom (Elettaria cardamomum) against Staphylococcus aureus and Proteus mirabilis. Tikrit J Pure Sci 17:14–18
Majdalawieh AF, Carr RI (2010) In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum). J Med Food 13:371–381. https://doi.org/10.1089/jmf.2009.1131
Aghasi M, Koohdani F, Qorbani M, Nasli-Esfahani E, Ghazi-Zahedi S, Khoshamal H, Keshavarz A, Sotoudeh G (2019) Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: a randomized double-blind placebo controlled clinical trial. J Sci Food Agric 99:3933–3940. https://doi.org/10.1002/jsfa.9617
Daneshi-Maskooni M, Keshavarz SA, Qorbani M, Mansouri S, Alavian SM, Badri-Fariman M, Jazayeri-Tehrani SA, Sotoudeh G (2019) Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: a double-blind randomized placebo-controlled clinical trial. BMC Compl Altern Med 19:59. https://doi.org/10.1186/s12906-019-2465-0
Kanthlal SK, Joseph J, Paul B (2020) Antioxidant and vasorelaxant effects of aqueous extract of large cardamom in L-NAME induced hypertensive rats. Clin Exp Hypertens 42:581–589. https://doi.org/10.1080/10641963.2020.1739699
Abu Gazia M, El-Magd MA (2018) Ameliorative effect of cardamom aqueous extract on doxorubicin-induced cardiotoxicity in rats. Cells Tissues Organs 206:62–72. https://doi.org/10.1159/000496109
Suneetha WJ, Krishnakantha TP (2005) Cardamom extract as inhibitor of human platelet aggregation. Phytother Res 19:437–440. https://doi.org/10.1002/ptr.1681
Aboubakr M, Abdelazem A (2015) Hepatoprotective effect of aqueous extract of cardamom against gentamicin induced hepatic damage in rats. Int J Sci Basic Appl 5:1–4. https://doi.org/10.14419/ijbas.v5i1.5435
Elkomy A, Aboubakr M, Elsawaf N (2015) Renal protective effect of cardamom against nephrotoxicity induced by gentamicin in rats. BVMJ 29:100–105
Ahmed AS, Ahmed Q, Saxena AK, Jamal P (2017) Evaluation of in vitro antidiabetic and antioxidant characterizations of Elettaria cardamomum (L) Maton (Zingiberaceae), Piper cubeba L f (Piperaceae), and Plumeria rubra L (Apocynaceae). Pak J Pharm Sci 30:113–126
Al-Youssef H, Alqahtani A, Hassan W, Alzoubi A, Abdelaziz S (2021) chemical profile, in vitro antioxidant, pancreatic lipase, and alpha-amylase inhibition assays of the aqueous extract of Elettaria cardamomum L. Fruits J Chem 2021:5583001. https://doi.org/10.1155/2021/5583001
Suddek GM (2014) Protective role of thymoquinone against liver damage induced by tamoxifen in female rats. Can J Physiol Pharmacol 92:640–644. https://doi.org/10.1139/cjpp-2014-0148
Albukhari AA, Gashlan HM, El-Beshbishy HA, Nagy AA, Abdel-Naim AB (2009) Caffeic acid phenethyl ester protects against tamoxifen-induced hepatotoxicity in rats. Food Chem Toxicol 47:1689–1695. https://doi.org/10.1016/j.fct.2009.04.021
El-Kashef DH, El-Sheakh AR (2019) Hepatoprotective effect of celecoxib against tamoxifen-induced liver injury via inhibiting ASK-1/JNK pathway in female rats. Life Sci 231:116573. https://doi.org/10.1016/j.lfs.2019.116573
Alshanwani AR, Mohamed AM, Faddah LM, Shaheen S, Arafah MM, Hagar H, Alhusaini AM, Alharbi FMB, AlHarthii A, Badr AM (2021) Cyanocobalamin and/or calcitriol mitigate renal damage-mediated by tamoxifen in rats: Implication of caspase-3/NF-κB signaling pathways. Life Sci 277:119512. https://doi.org/10.1016/j.lfs.2021.119512
Hickman DL (2019) Wellbeing of alcohol-preferring rats euthanized with carbon dioxide at very low and low volume displacement rates. J Am Assoc Lab Anim Sci 58:78–82. https://doi.org/10.30802/AALAS-JAALAS-18-000004
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78. https://doi.org/10.1016/0304-4165(79)90289-7
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-x
Su S, Liang T, Zhou X, He K, Li B, Xia X (2019) Qingyi decoction attenuates severe acute pancreatitis in rats via inhibition of inflammation and protection of the intestinal barrier. J Int Med Res 47:2215–2227. https://doi.org/10.1177/0300060518809289
Gan SI, Edwards AL, Symonds CJ, Beck PL (2006) Hypertriglyceridemia-induced pancreatitis: A case-based review. World J Gastroenterol 12:7197–7202. https://doi.org/10.3748/wjg.v12.i44.7197
Ewald N, Hardt PD, Kloer HU (2009) Severe hypertriglyceridemia and pancreatitis: presentation and management. Curr Opin Lipidol 20:497–504. https://doi.org/10.1097/MOL.0b013e3283319a1d
Han X, Shen T, Lou H (2007) Dietary polyphenols and their biological significance. Int J Mol Sci 8:950–988. https://doi.org/10.3390/i8090950
Brglez Mojzer E, Knez Hrnčič M, Škerget M, Knez Ž, Bren U (2016) Polyphenols: extraction methods, antioxidative action, bioavailability and Anticarcinogenic effects. Molecules 21:901. https://doi.org/10.3390/molecules21070901
Miltonprabu S, Thangapandiyan S (2015) Epigallocatechin gallate potentially attenuates Fluoride induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. J Trace Elem Med Biol 29:321–335. https://doi.org/10.1016/j.jtemb.2014.08.015
Liu CL, Yang TL (2003) Sequential changes in serum triglyceride levels during adjuvant tamoxifen therapy in breast cancer patients and the effect of dose reduction. Breast Cancer Res Treat 79:11–16. https://doi.org/10.1023/a:1023348021773
Filippatos TD, Liberopoulos EN, Pavlidis N, Elisaf MS, Mikhailidis DP (2009) Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat Rev 35:175–184. https://doi.org/10.1016/j.ctrv.2008.09.007
Galleano M, Calabro V, Prince PD, Litterio MC, Piotrkowski B, Vazquez-Prieto MA, Miatello RM, Oteiza PI, Fraga CG (2012) Flavonoids and metabolic syndrome. Ann N Y Acad Sci 1259:87–94. https://doi.org/10.1111/j.1749-6632.2012.06511.x
Wynne K, Devereaux B, Dornhorst A (2019) Diabetes of the exocrine pancreas. J Gastroenterol Hepatol 34:346–354. https://doi.org/10.1111/jgh.14451
Mega C, de Lemos ET, Vala H, Fernandes R, Oliveira J, Mascarenhas-Melo F, Teixeira F, Reis F (2011) Diabetic nephropathy amelioration by a low-dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes Res 2011:162092. https://doi.org/10.1155/2011/162092
Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS (2007) Regulation of lipolysis in adipocytes. Annu Rev Nutr 27:79–101. https://doi.org/10.1146/annurev.nutr.27.061406.093734
Nitasha Bhat GM, Nayak N, Vinodraj K, Chandralekha N, Mathai P, Cherian J (2015) Comparison of the efficacy of cardamom (Elettaria cardamomum) with pioglitazone on dexamethasone-induced hepatic steatosis, dyslipidemia, and hyperglycemia in albino rats. J Adv Pharm Technol Res 6:136–140. https://doi.org/10.4103/2231-4040.157981
Winarsi H, Sasongko N, Purwanto A, Nuraeni I (2014) Effect of cardamom leaves extract as antidiabetic, weight lost and hypocholesterolemic to alloxan induced Sprague Dawley diabetic rats. Int Food Res J 21:2253
Soares JMD, Pereira Leal AEB, Silva JC, Almeida JRGS, de Oliveira HP (2017) Influence of Flavonoids on Mechanism of Modulation of Insulin Secretion. Pharmacogn Mag 13:639–646. https://doi.org/10.4103/pm.pm_87_17
Oliveira H, Fernandes A, Brás N, Mateus N, de Freitas V, Fernandes I (2020) Anthocyanins as antidiabetic agents-in vitro and in silico approaches of preventive and therapeutic effects. Molecules 25:3813. https://doi.org/10.3390/molecules25173813
Kim HK, Park HR, Lee JS, Chung TS, Chung HY, Chung J (2007) Down-regulation of iNOS and TNF-alpha expression by kaempferol via NF-kappaB inactivation in aged rat gingival tissues. Biogerontology 8:399–408. https://doi.org/10.1007/s10522-007-9083-9
Sun J, Bhatia M (2007) Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 292:G143–G153. https://doi.org/10.1152/ajpgi.00271.2006
Seo SW, Jung WS, Lee SE, Choi CM, Shin BC, Kim EK, Kwon KB, Hong SH, Yun KJ, Park RK, Shin MK, Song HJ, Park SJ (2008) Effects of bee venom on cholecystokinin octapeptide-induced acute pancreatitis in rats. Pancreas 36:e22–e29. https://doi.org/10.1097/MPA.0b013e31815a396b
Hong YP, Yu J, Su YR, Mei FC, Li M, Zhao KL, Zhao L, Deng WH, Chen C, Wang WX (2020) high-fat diet aggravates acute pancreatitis via TLR4-mediated necroptosis and inflammation in rats. Oxid Med Cell Longev 2020:8172714. https://doi.org/10.1155/2020/8172714
Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E (2007) Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm 2007:45673. https://doi.org/10.1155/2007/45673
Kawai T, Akira S (2005) Pathogen recognition with Toll-like receptors. Curr Opin Immunol 17:338–344. https://doi.org/10.1016/j.coi.2005.02.007
Abdelmageed ME, Nader MA, Zaghloul MS (2021) Targeting HMGB1/TLR4/NF-κB signaling pathway by protocatechuic acid protects against l-arginine induced acute pancreatitis and multiple organs injury in rats. Eur J Pharmacol 906:174279. https://doi.org/10.1016/j.ejphar.2021.174279
Junyuan Z, Hui X, Chunlan H, Junjie F, Qixiang M, Yingying L, Lihong L, Xingpeng W, Yue Z (2018) Quercetin protects against intestinal barrier disruption and inflammation in acute necrotizing pancreatitis through TLR4/MyD88/p38 MAPK and ERS inhibition. Pancreatol 18:742–752. https://doi.org/10.1016/j.pan.2018.08.001
Ma R, Yuan F, Wang S, Liu Y, Fan T, Wang F (2018) Calycosin alleviates cerulein-induced acute pancreatitis by inhibiting the inflammatory response and oxidative stress via the p38 MAPK and NF-κB signal pathways in mice. Biomed Pancreatology 105:599–605. https://doi.org/10.1016/j.biopha.2018.05.080
Jakkampudi A, Jangala R, Reddy BR, Mitnala S, Nageshwar Reddy D, Talukdar R (2016) NF-κB in acute pancreatitis: Mechanisms and therapeutic potential. Pancreatology 16:477–488. https://doi.org/10.1016/j.pan.2016.05.001
Chen Z, Chen Y, Pan L, Li H, Tu J, Liu C, Dai X, Zhang X, Sun G, Feng D (2015) Dachengqi decoction attenuates inflammatory response via inhibiting HMGB1 mediated NF-κB and P38 MAPK signaling pathways in severe acute pancreatitis. Cell Physiol Biochem 37:1379–1389. https://doi.org/10.1159/000430403
Souissi M, Azelmat J, Chaieb K, Grenier D (2020) Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe 61:102089. https://doi.org/10.1016/j.anaerobe.2019.102089
Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, Partecke LI, Döring P, Gukovsky I, Gukovskaya AS, Wagh PR, Lerch MM, Mayerle J (2018) Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology 154:704–718.e10. https://doi.org/10.1053/j.gastro.2017.10.018
Al-Hassan S, Attia H, Alomar H, Arafa M, Ali RA (2021) The inhibitory mechanisms of losartan and vitamin D on amiodarone-induced lung inflammation in rats: Role of mitogen-activated protein kinases/activator protein-1. J Biochem Mol Toxicol 35:e22923. https://doi.org/10.1002/jbt.22923
Tsukamoto K, Kinoshita M, Kojima K, Mikuni Y, Kudo M, Mori M, Fujita M, Horie E, Shimazu N, Teramoto T (2002) Synergically increased expression of CD36, CLA-1 and CD68, but not of SR-A and LOX-1, with the progression to foam cells from macrophages. J Atheroscler Thromb 9:57–64. https://doi.org/10.5551/jat.9.57
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Adewole SO, Caxton-Martins EA, Ojewole JA (2006) Protective effect of quercetin on the morphology of pancreatic beta-cells of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med 4:64–74. https://doi.org/10.4314/ajtcam.v4i1.31196
Lee YJ, Suh KS, Choi MC, Chon S, Oh S, Woo JT, Kim SW, Kim JW, Kim YS (2010) Kaempferol protects HIT-T15 pancreatic beta cells from 2-deoxy-D-ribose-induced oxidative damage. Phytother Res 24:419–423. https://doi.org/10.1002/ptr.2983
Ghorbani A, Rashidi R, Shafiee-Nick R (2019) Flavonoids for preserving pancreatic beta cell survival and function: A mechanistic review. Biomed Pharmacother 111:947–957. https://doi.org/10.1016/j.biopha.2018.12.127
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res 51:117–123. https://doi.org/10.1016/j.phrs.2004.06.002
Bashir N, Manoharan V, Miltonprabu S (2016) Grape seed proanthocyanidins protects against cadmium induced oxidative pancreatitis in rats by attenuating oxidative stress, inflammation and apoptosis via Nrf-2/HO-1 signaling. J Nutr Biochem 32:128–141. https://doi.org/10.1016/j.jnutbio.2016.03.001
Du D, Yao L, Zhang R, Shi N, Shen Y, Yang X, Zhang X, Jin T, Liu T, Hu L, Xing Z, Criddle DN, Xia Q, Huang W, Sutton R (2018) Protective effects of flavonoids from Coreopsis tinctoria Nutt. on experimental acute pancreatitis via Nrf-2/ARE-mediated antioxidant pathways. J Ethnopharmacol 224:261–272. https://doi.org/10.1016/j.jep.2018.06.003
Acknowledgements
Authors extend their appreciation to Prince Naif Health Research Center, Investigator support Unit for the language editing service provided.
Funding
The authors extend their appreciation to the Deputyship for Research and Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-154–1).
Author information
Authors and Affiliations
Contributions
HA, AA, and NA-a contributed to the study conception and design. All authors contributed to methodology, investigations and data analysis. HA wrote the manuscript. Resources were supplied by HA, AA and RM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.

43188_2023_198_MOESM1_ESM.jpg
Supplementary file1 Supplementary file 1: Morphology of the pancreas from the rats in the normal control and tamoxifen (TAM)-intoxicated groups, and from rats treated with 4, 6, 8, 10, and 12 mL/kg of the cardamom aqueous extract (CAE). A: Normal control; B: TAM-intoxicated rats; C: TAM+ CAE (4 mL/kg); D: TAM+ CAE (6 mL/kg); E: TAM+ CAE (8 mL/kg); F: TAM+ CAE (10 mL/kg); G: TAM+ CAE (12 mL/kg) (JPG 36 KB)

43188_2023_198_MOESM3_ESM.jpg
Supplementary file3 Photomicrographs of the rat pancreas stained with hematoxylin and eosin. Scale bar; 100μm. Effect of different doses of the cardamom aqueous extract (CAE) on the histological changes following tamoxifen (TAM) intoxication (A) Section of the pancreas from control rat showing normal histological features of pancreatic acini (long arrows), normal multiple islets of Langerhans (stars) and normal thin slit-like septa (short arrows). (B) Pancreatic section from rat that received TAM showing marked depletion of pancreatic acini (long arrows). The acini were atrophic with degeneration of their lining epithelium, and there were no prominent islets of Langerhans. A marked increase in the thickness of the connective tissue septa (short arrows) infiltrated with many inflammatory cells was observed. (C) & (D) Sections of the pancreas from rats that concomitantly received 4 or 6 mL/kg CAE, respectively, showing histopathological changes mimicking the TAM-intoxicated group. (E) Pancreatic section from rat that received 8 mL/kg CAE +TAM showing the appearance of many acini and decreased connective tissue septa (short arrows) compared with that in the 4 and 6 mL/kg groups. (F) & (G) Sections of the pancreas from rats that received 10 and 12 mL/kg CAE + TAM showing an increase in the number of islets of Langerhans (stars) and acini (long arrows), with a marked decrease in connective tissue septa (short arrows) particularly at 10 mL/kg. (JPG 200 KB)

43188_2023_198_MOESM4_ESM.jpg
Supplementary file4 Supplementary file 4. Effect of the different doses of the cardamom aqueous extract (CAE) on the levels of serum α-amylase, pancreatic lipase, triglycerides (TG) and glucose in tamoxifen (TAM)-intoxicated female rats. a: significant difference vs normal control, b: Significant difference vs. TAM-intoxicated group, c and d: Significant differences vs. 10 and 12 ml/kg-treated groups, respectively. ***p < 0.001, ** p < 0.01, * p < 05. (JPG 128 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Attia, H., Alzoubi, A., Al-anazi, N. et al. Protective effects of cardamom aqueous extract against tamoxifen-induced pancreatic injury in female rats. Toxicol Res. 39, 721–737 (2023). https://doi.org/10.1007/s43188-023-00198-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-023-00198-w