Abstract
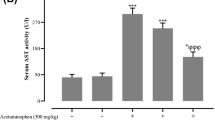
Drug induced liver injury (DILI) is a global issue and acetaminophen (APAP) is considered as the main cause of this. Due to increasing incidents of DILI, current study attempted to investigate an alternative but better role of terazosin (alpha-adrenergic blocker) in APAP-induced acute liver injury in an animal model using New Zealand rabbits. APAP (1 g/kg of body weight) was given to New Zealand rabbits either with or without terazosin (0.5 mg/kg) and serum was collected after 4 h. Serum alanine transaminase (ALT), alkaline phosphatase (ALP) and ferritin level were determined to analyze the liver functioning of treated rabbits. Furthermore, total cholesterol (TC), total lipids (TL), high-density lipoproteins (HDL), low-density lipoprotein (LDL) and triglycerides (TG) levels were estimated to find any change in lipid profile of the treated animals. Moreover, the urea and creatinine levels assayed the actual renal functionality. To identify any modification in gene expression, qPCR of cytochrome P2E1 (CYP2E1) was performed. Terazosin in combination with APAP enhanced liver functioning by reducing the levels of liver injury markers viz. ALP and ALT, while lipid profile was also lowered by down regulation of TC, TL, LDL and TG with enhanced HDL levels. It caused significant down regulation of expression level of CYP2E1. It is concluded that terazosin has better effects induced on the recovery of normal liver functioning, by improving the liver profile, lipid profile and renal functioning both at tissue and molecular levels.




Similar content being viewed by others
References
Zhang A, Sun H, Wang X (2013) Recent advances in natural products from plants for treatment of liver diseases. Eur J Med Chem 63:570–577. https://doi.org/10.1016/j.ejmech.2012.12.062
Bernal W, Wendon J (2013) Acute liver failure. N Engl J Med 369:2525–2534. https://doi.org/10.1056/nejmra1208937
Vuppalanchi R, Liangpunsakul S, Chalasani N (2007) Etiology of new-onset jaundice: how often is it caused by idiosyncratic drug-induced liver injury in the United States? Am J Gastroenterol 102:558–562. https://doi.org/10.1111/j.1572-0241.2006.01019.x
Teschke R, Frenzel C, Wolff A, Eickhoff A, Schulze J (2014) Drug induced liver injury: accuracy of diagnosis in published reports. Ann Hepatol 13:248–255. https://doi.org/10.1016/S1665-2681(19)30888-9
Vliegenthart ADB, Tucker CS, Del Pozo J, Dear JW (2014) Zebrafish as model organisms for studying drug-induced liver injury. Br J Clin Pharmacol 78:1217–1227. https://doi.org/10.1111/bcp.12408
Hussaini SH, Farrington EA (2007) Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf 6:673–684. https://doi.org/10.1517/14740338.6.6.673
Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ, Murray NG, McCashland T, Reisch JS, Robuck PR (2009) Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 137:856-864.e851. https://doi.org/10.1053/j.gastro.2009.06.006
Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, DGEM, Ferenci P, Holm E, Dahl SV, Müller MJ, Nolte W, ESPEN (2006) ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 25:285–294. https://doi.org/10.1016/j.clnu.2006.01.018
Kheradpezhouh E, Panjehshahin MR, Miri R, Javidnia K, Noorafshan A, Monabati A, Dehpour AR (2010) Curcumin protects rats against acetaminophen-induced hepatorenal damages and shows synergistic activity with N-acetylcysteine. Eur J Pharmacol 628:274–281. https://doi.org/10.1016/j.ejphar.2009.11.027
Yang R, Miki K, He X, Killeen ME, Fink MP (2009) Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit Care 13:R55. https://doi.org/10.1186/cc7782
Panackel C, Thomas R, Sebastian B, Mathai S (2015) Recent advances in management of acute liver failure. Indian J Crit Care Med 19:27. https://doi.org/10.4103/0972-5229.148636
Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA (2002) Recent patterns of medication use in the ambulatory adult population of the United States. JAMA 287:337. https://doi.org/10.1001/jama.287.3.337
McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H (2012) The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 122:1574–1583. https://doi.org/10.1172/jci59755
Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–328. https://doi.org/10.1093/toxsci/67.2.322
Kon K, Kim JS, Jaeschke H, Lemasters JJ (2004) Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 40:1170–1179. https://doi.org/10.1002/hep.20437
Masubuchi Y, Suda C, Horie T (2005) Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol 42:110–116. https://doi.org/10.1016/j.jhep.2004.09.015
Sadiq F, Channa IS, Safdar M, Junejo Y, Khailany RA, Ahktar MA, Saeed M, Babar N, Babar ME, Ozaslan M, Gondal MA (2019) Hepatoprotective effects of arabica coffee beans in paracetamol induced hepatotoxic animal models. Int J Biosci 15:340–351. https://doi.org/10.12692/ijb/15.1.340-351
Liudmila LM, Katrin S, Caroline FT, Garret AF, Russ BA, Teri EK (2015) PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses. Pharmacogenet Genomics 25:416–426. https://doi.org/10.1097/FPC.0000000000000150
Katzung BG, Masters S, Trevor AJ (2012) Basic and clinical pharmacology (LANGE Basic Science). McGraw-Hill Education, New York
Christensen M, Bruskewitz R (1990) Clinical manifestations of benign prostatic hyperplasia and indications for therapeutic intervention. Urol Clin N Am 17:509–516. https://doi.org/10.1016/S0094-0143(21)00964-2
Ernst E, Pittler MH (1998) Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol 159:433–436. https://doi.org/10.1016/s0022-5347(01)63942-9
Messerli FH (2001) Doxazosin and congestive heart failure. J Am Coll Cardiol 38:1295–1296. https://doi.org/10.1016/S0735-1097(01)01534-0
Khoury AF (1991) α-Blocker therapy of hypertension. JAMA 266:394. https://doi.org/10.1001/jama.1991.03470030094030
Conour LA, Murray KA, Brown MJ (2006) Preparation of animals for research–issues to consider for rodents and rabbits. ILAR J 47:283–293. https://doi.org/10.1093/ilar.47.4.283
Ahmed J (2014) A significant hepatotoxicity associated with paracetamol overdose in the absence of kidney injury in rabbits. Int J Basic Clin Pharmacol 3:1043–1047. https://doi.org/10.5455/2319-2003.ijbcp20141216
Sabina EP, Pragasam SJ, Kumar S, Rasool M (2011) 6-Gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. Zhong Xi Yi Jie He Xue Bao 9:1264–1269. https://doi.org/10.3736/jcim20111116
Chen G, Liu H, Cheng F (2013) Fangjihuangqi Tang improved lower urinary tract dysfunction in benign prostatic hyperplasia rats model. J Tradit Chin Med 33:349–354. https://doi.org/10.1016/s0254-6272(13)60177-6
Burtis CA, Ashwood ER, Bruns DE (2012) Preface tietz textbook of clinical chemistry and molecular diagnostics. Elsevier, Amsterdam, p xvi
Zilva JF, Pannall PR, Mayne PD (1975) Clinical chemistry in diagnosis and treatment. Lloyd-Luke, London
Talke H, Schubert G (1965) Enzymatische harnstoffbestimmung in blut und serum im optischen test nachwarburg. J Mol Med 43:174–175. https://doi.org/10.1007/BF01484513
Ishitsuka Y, Kondo Y, Kadowaki D (2020) Toxicological property of acetaminophen: the dark side of a safe antipyretic/analgesic drug? Biol Pharm Bull 43:195–206. https://doi.org/10.1248/bpb.b19-00722
Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA (2008) Guidelines for the management of paracetamol poisoning in Australia and New Zealand-explanation and elaboration. Med J Aust 188:296. https://doi.org/10.5694/j.1326-5377.2008.tb01625.x
Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, Guarner C, Forné M, Solà R, Castellote J, Rigau J, Laporte J-R (2011) Paracetamol in therapeutic dosages and acute liver injury: causality assessment in a prospective case series. BMC Gastroenterol 11:1. https://doi.org/10.1186/1471-230x-11-80
Rehman JU, Aktar N, Khan MY, Ahmad K, Ahmad M, Sultana S, Asif HM (2015) Phytochemical screening and hepatoprotective effect of Alhagi maurorum boiss (leguminosae) against paracetamol-induced hepatotoxicity in rabbits. Trop J Pharm Res 14:1029. https://doi.org/10.4314/tjpr.v14i6.13
Anastasiou OE, Kälsch J, Hakmouni M, Kucukoglu O, Heider D, Korth J, Manka P, Sowa J, Bechmann L, Saner FH, Paul A, Gerken G, Baba HA, Canbay A (2017) Low transferrin and high ferritin concentrations are associated with worse outcome in acute liver failure. Liver Int 37:1032–1041. https://doi.org/10.1111/liv.13369
Prieto J, Barry M, Sherlock S (1975) Serum ferritin in patients with iron overload and with acute and chronic liver diseases. Gastroenterology 68:525–533. https://doi.org/10.1016/S0016-5085(75)80092-8
Madi Almajwal A, Farouk Elsadek M (2015) Lipid-lowering and hepatoprotective effects of vitis viniferadried seeds on paracetamol-induced hepatotoxicity in rats. Nutr Res Pract 9:37. https://doi.org/10.4162/nrp.2015.9.1.37
Rifkind BM (1984) Lipid research clinics coronary primary prevention trial: results and implications. Am J Cardiol 54:30–34. https://doi.org/10.1016/0002-9149(84)90854-3
Castelli WP, Doyle JT, Gordon T, Hames CG, Hjortland MC, Hulley SB, Kagan A, Zukel WJ (1977) HDL cholesterol and other lipids in coronary heart disease. The cooperative lipoprotein phenotyping study. Circulation 55:767–772. https://doi.org/10.1161/01.cir.55.5.767
Deger G (1986) Effect of terazosin on serum lipids. Am J Med 80:82–85. https://doi.org/10.1016/0002-9343(86)90858-2
Leren P, Helgeland A, Holme I, Foss PO, Hjermann I, Lund-Larsen PG (1980) Effect of propranolol and prazosin on blood lipids. Lancet 316:4–6. https://doi.org/10.1016/s0140-6736(80)92888-3
Kher K, Makker S (1987) Acute renal failure due to acetaminophen ingestion without concurrent hepatotoxicity. Am J Med 82:1280–1281. https://doi.org/10.1016/0002-9343(87)90254-3
Thomas SHL (1993) Paracetamol (acetaminophen) poisoning. Pharmacol Ther 60:91–120. https://doi.org/10.1016/0163-7258(93)90023-7
Pedraza-Chaverrı́ J, Barrera D, Hernández-Pando R, Medina-Campos ON, Cruz C, Murguı́a F, Juárez-Nicolás C, Correa-Rotter R, Torres N, Tovar AR (2004) Soy protein diet ameliorates renal nitrotyrosine formation and chronic nephropathy induced by puromycin aminonucleoside. Life Sci 74:987–999. https://doi.org/10.1016/j.lfs.2003.07.045
Yang D, Lin S, Yang D, Wei L, Shang W (2012) Effects of short- and long-term hypercholesterolemia on contrast-induced acute kidney injury. Am J Nephrol 35:80–89. https://doi.org/10.1159/000335077
Lee SST, Buters JTM, Pineau T, Fernandez-Salguero P, Gonzalez FJ (1996) Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem 271:12063–12067. https://doi.org/10.1074/jbc.271.20.12063
Bièche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I (2007) Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics 17:731–742. https://doi.org/10.1097/fpc.0b013e32810f2e58
Prasad JS, Chen N, YuXlu L, Goon DJW, Holtzman JL (1990) Effects of ethanol and inhibitors on the binding and metabolism of acetaminophen and N-acetyl-p-benzoquinone imine by hepatic microsomes from control and ethanol-treated rats. Biochem Pharmacol 40:1989–1995. https://doi.org/10.1016/0006-2952(90)90228-d
Albillos A, Lledó JL, Rossi I, Pérez-Páramo M, Tabuenca MJ, Bañares R, Iborra J, Garrido A, Escartín P, Bosch J (1995) Continuous prazosin administration in cirrhotic patients: effects on portal hemodynamics and on liver and renal function. Gastroenterology 109:1257–1265. https://doi.org/10.1016/0016-5085(95)90586-3
Randle LE, Sathish JG, Kitteringham NR, Macdonald I, Williams DP, Park BK (2008) α1-Adrenoceptor antagonists prevent paracetamol-induced hepatotoxicity in mice. Br J Pharmacol 153:820–830. https://doi.org/10.1038/sj.bjp.0707620
Funding
The study was supported by departmental research grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Hashmat, Z., Channa, I.S., Safdar, M. et al. Adrenergic blocker terazosin potentially suppresses acetaminophen induced-acute liver injury in animal models via CYP2E1 gene. Toxicol Res. 38, 323–330 (2022). https://doi.org/10.1007/s43188-021-00116-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-021-00116-y