Abstract
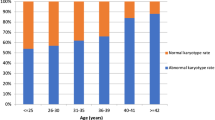
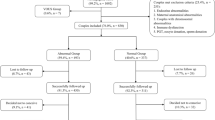
The objective of this study was to characterize the relationship between embryonic chromosomal errors in the products of conception (POC) and maternal age, gestational age (GA) of pregnancy loss, and findings on routine recurrent pregnancy loss (RPL) workup. This is a retrospective cohort study of women with a history of ≥ 2 pregnancy losses and who underwent cytogenetic testing on the POC of a subsequent pregnancy loss at an academic tertiary RPL referral center. The association between the odds of embryonic chromosomal errors in POC and maternal age, GA of pregnancy loss, as well as RPL work up findings was investigated. A total of 1107 miscarriages were analyzed from 741 women. There was an overall linear relationship between embryonic chromosomal errors and maternal age, with a nearly twofold increase in the odds of chromosomal error with every 5-year increase in maternal age (P < 0.0001). The association between chromosomal errors and GA was also linear (P = 0.0001), with most losses having no chromosomal errors after 13 weeks’ gestation. Women with ≥ 1 positive findings on routine RPL diagnostic workup had lower odds of embryonic chromosomal errors compared to those with a normal workup [OR 0.57 (95% CI = 0.41–0.80)]. Notably, the estimated prevalence of chromosomal error remained high (> 60%) in women ≥ 35 years old irrespective of findings on routine evaluation. While embryonic chromosomal errors were associated with advanced maternal age, early GA of loss, and a negative routine RPL evaluation, the prevalence of chromosomal errors remained high in all subpopulations. These findings suggest that primary cytogenetic testing on POCs should be offered at the time of second and subsequent pregnancy losses in all RPL patients.





Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Stirrat GM. Recurrent miscarriage. Lancet. 1990;336(8716):673–5.
Chester MR, Tirlapur A, Jayaprakasan K. Current management of recurrent pregnancy loss. Obstet Gynaecol. 2022;24(4):260–71.
Practice Committee of the American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril. 2013;99(1):63.
Bender Atik R, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):hoy004.
Royal College of Obstetricians and Gynaecologists. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. In: Green-top Guideline No. 17. London: RCOG; 2011.
Stephenson M, Kutteh W. Evaluation and management of recurrent early pregnancy loss. Clin Obstet Gynecol. 2007;50(1):132–45.
Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril. 1996;66(1):24–9.
Jaslow CR, Carney JL, Kutteh WH. Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil Steril. 2010;93(4):1234–43.
Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11.
Bernardi LA, Plunkett BA, Stephenson MD. Is chromosome testing of the second miscarriage cost saving? A decision analysis of selective versus universal recurrent pregnancy loss evaluation. Fertil Steril. 2012;98(1):156–61.
Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. 2018;33(4):579–87.
Dahdouh EM, Kutteh WH. Genetic testing of products of conception in recurrent pregnancy loss evaluation. Reprod Biomed Online. 2021;43(1):120–6.
Johnson DS, et al. Preclinical validation of a microarray method for full molecular karyotyping of blastomeres in a 24-h protocol. Hum Reprod. 2010;25(4):1066–75.
Chambers JM, Hastie TJ. Statistical models in S. Pacific Grove: Wadsworth & Brooks/Cole; 1992.
Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29(4):1165–88.
Franasiak JM, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-663.e1.
Tsutsumi M, et al. Age-related decrease of meiotic cohesins in human oocytes. PLoS ONE. 2014;9(5):e96710.
Duncan FE, et al. Chromosome cohesion decreases in human eggs with advanced maternal age. Aging Cell. 2012;11(6):1121–4.
Shimoi G, et al. Destabilization of spindle assembly checkpoint causes aneuploidy during meiosis II in murine post-ovulatory aged oocytes. J Reprod Dev. 2019;65(1):57–66.
Huo P, et al. High accuracy of quantitative fluorescence polymerase chain reaction combined with non-invasive pre-natal testing for mid-pregnancy diagnosis of common fetal aneuploidies: a single-center experience in China. Exp Ther Med. 2019;18(1):711–21.
Mehta GD, Agarwal M, Ghosh SK. Functional characterization of kinetochore protein, Ctf19 in meiosis I: an implication of differential impact of Ctf19 on the assembly of mitotic and meiotic kinetochores in Saccharomyces cerevisiae. Mol Microbiol. 2014;91(6):1179–99.
Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–91.
Simpson J, Carson S. Genetic and nongenetic causes of pregnancy loss. The Global Library of Women’s Medicine. 2013. https://doi.org/10.3843/GLOWM.10319
Soler A, et al. Overview of chromosome abnormalities in first trimester miscarriages: a series of 1,011 consecutive chorionic villi sample karyotypes. Cytogenet Genome Res. 2017;152(2):81–9.
Menasha J, et al. Incidence and spectrum of chromosome abnormalities in spontaneous abortions: new insights from a 12-year study. Genet Med. 2005;7(4):251–63.
Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet. 1985;70(1):11–7.
Warburton D, et al. Monosomy X: a chromosomal anomaly associated with young maternal age. Lancet. 1980;1(8161):167–9.
Terry J. The value of ancillary testing in amniotic fluid infection/inflammation-related early pregnancy loss and perinatal death in British Columbia. BC Med J. 2021;63(9):383–7.
Qi ST, et al. Arrested human embryos are more likely to have abnormal chromosomes than developing embryos from women of advanced maternal age. J Ovarian Res. 2014;7:65.
Wapner RJ, Lewis D. Genetics and metabolic causes of stillbirth. Semin Perinatol. 2002;26(1):70–4.
Acknowledgements
The Division of Reproductive Endocrinology and Infertility at the University of British Columbia supported the authors throughout the study period and manuscript preparation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Korkidakis, A., Albert, A.Y., Jiang, I. et al. The Clinical Significance of Embryonic Chromosomal Errors in Recurrent Pregnancy Loss: an Analysis of 1107 Miscarriages. Reprod. Sci. 30, 3019–3026 (2023). https://doi.org/10.1007/s43032-023-01239-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01239-3