Abstract
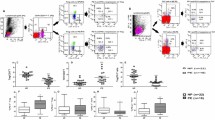
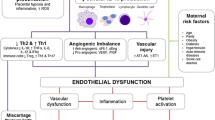
The disturbance of maternofetal immune tolerance is identified as one of the important issues in the pathology of preeclampsia (PE). PE exosomes are believed to possess significant roles in immune abnormalities. In this study, to assess the possible effects of PE exosomes in the pathophysiology of preeclampsia patients, exosomes were isolated from the serum of PE patients and incubated with peripheral blood mononuclear cells (PBMCs) of healthy pregnant women. Also, exosomes from healthy pregnant women were utilized as the control. Th17/Treg ratio in PE and healthy pregnant women and the effects of PE exosomes on expression level of Th17 and Treg transcription factors, as well as their related cytokines in PBMCs of healthy pregnant women, were evaluated. A significant decrease in Treg cell number and increase in Th17 cells and Th17/Treg ratio were observed in PE patients. Following PE-exosome intervention, a significant increase in mRNA expression level of RORγt, IL-17, IL-23, IL-1β, and IL-6, and significant decrease in IL-10 and TGFβ were evident. On the other hand, no significant difference in FoxP3 level was detected. Additionally, increased IL-6, IL-17, IL-23, and IL-1β levels and decreased IL-10 level in the supernatant of cultured PBMCs from healthy pregnant women following PE-exosome intervention were exhibited. However, TGF-β level did not change significantly. Based on our findings, PE exosomes are able to alter the activity of Th17 and Treg cells as well as their related gene expression and cytokine profiles. These findings support the probable role of PE exosomes in PE pathogenesis.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Code Availability
Not applicable.
Abbreviations
- PE:
-
Preeclampsia
- PBMCs:
-
Peripheral blood mononuclear cells
- Th:
-
T helper
- NKT:
-
Natural killer T cell
- RORγt:
-
Retinoic acid receptor–related orphan nuclear receptor γt
- FoxP3:
-
Fork-head box P3
- IL:
-
Interleukin
- TGF-β:
-
Transforming growth factor
- ELISA:
-
Enzyme-linked immunosorbent assay
- HRP:
-
Horseradish peroxidase
- AP:
-
Alkaline phosphatase
- PMA:
-
Phorbol myristate acetate
- cDNA:
-
Complementary DNA
References
Dahabiyeh LA, Tooth D, Kurlak LO, Mistry HD, Pipkin FB, Barrett DA. A pilot study of alterations in oxidized angiotensinogen and antioxidants in pre-eclamptic pregnancy. Sci Rep. 2020;10:1–8. https://doi.org/10.1038/s41598-020-58930-7.
Zheng L, Huang J, Su Y, Wang F, Kong H, Xin H. Overexpression of tissue factor pathway inhibitor 2 attenuates trophoblast proliferation and invasion in preeclampsia. Human Cell. 2020;33:512. https://doi.org/10.1007/s13577-020-00322-0.
Tian M, Zhang Y, Liu Z, Sun G, Mor G, Liao A. The PD-1/PD-L1 inhibitory pathway is altered in pre-eclampsia and regulates T cell responses in pre-eclamptic rats. Sci Rep. 2016;6:1–14. https://doi.org/10.1038/srep27683.
El-Chennawi F, Rageh IM, Mansour AI, Darwish MI, Elghzaly AA, Sakr BE, et al. Comparison of the percentages of CD4+ CD25high FOXP3+, and CD4+ CD25low FOXP3+, and CD4+ FOXP3+ Tregs, in the umbilical cord blood of babies born to mothers with and without preeclampsia. Am J Reprod Immunol. 2017;78:1–7. https://doi.org/10.1111/aji.12761.
Eghbal-Fard S, Yousefi M, Heydarlou H, Ahmadi M, Taghavi S, Movasaghpour A, Jadidi-Niaragh F, Yousefi B, Dolati S, Hojjat-Farsangi M, Rikhtegar R, Nouri M, Aghebati-Maleki L. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J Cell Physiol. 2019;234:5106–16. https://doi.org/10.1002/jcp.27315.
Kamrani A, Soltani-Zangbar MS, Shiri S, Yousefzadeh Y, Pourakbari R, Aghebati-Maleki L, Mehdizadeh A, Danaii S, Jadidi-Niaragh F, Yousefi B, Kafil HS, Hojjat-Farsangi M, Motavalli R, Zolfaghari M, Haji-Fatahaliha M, Mahmoodpoor A, Ahmadian Heris J, Emdadi A, Yousefi M. TIGIT and CD155 as immune-modulator receptor and ligand on CD4+ T cells in preeclampsia patients. Immunological Investigations. 2021;1023–38. https://doi.org/10.1080/08820139.2021.1904976
Soltani-Zangbar MS, Pahlavani B, Zolghadri J, Gharesi-Fard B. Angiotensin type 2 receptor gene polymorphisms and susceptibility to preeclampsia. J Reprod Infertil. 2018;19:95–99. http://www.ncbi.nlm.nih.gov/pubmed/30009143. Accessed 22 March 2021
Toldi G, Saito S, Shima T, Halmos A, Veresh Z, Vásárhelyi B, Rigós Jr J, Molvarec A. The frequency of peripheral blood CD4+ CD25high FoxP3+ and CD4+ CD25− FoxP3+ regulatory T cells in normal pregnancy and pre-eclampsia. Am J Reprod Immunol. 2012;68:175–80. https://doi.org/10.1111/j.1600-0897.2012.01145.x.
Azizieh F, Raghupathy R, Makhseed M. Maternal cytokine production patterns in women with pre-eclampsia. Am J Reprod Immunol. 2005;54:30–7. https://doi.org/10.1111/j.1600-0897.2005.00278.x.
Hashemi V, Dolati S, Hosseini A, Gharibi T, Danaii S, Yousefi M. Natural killer T cells in preeclampsia: an updated review. Biomed Pharmacother. 2017;95:412–8. https://doi.org/10.1016/j.biopha.2017.08.077.
Hosseini A, Dolati S, Hashemi V, Abdollahpour-Alitappeh M, Yousefi M. Regulatory T and T helper 17 cells: their roles in preeclampsia. J Cell Physiol. 2018;233:6561–73. https://doi.org/10.1002/jcp.26604.
Li J, Huang L, Wang S, Zhang Z. The prevalence of regulatory T and dendritic cells is altered in peripheral blood of women with pre-eclampsia. Pregnancy Hypertens. 2019;17:233–40. https://doi.org/10.1016/j.preghy.2019.07.003.
Chiarello DI, Salsoso R, Toledo F, Mate A, Vázquez CM, Sobrevia L. Foetoplacental communication via extracellular vesicles in normal pregnancy and preeclampsia. Mol Aspects Med. 2018;60:69–80. https://doi.org/10.1016/j.mam.2017.12.002.
Pourakbari R, Khodadadi M, Aghebati-Maleki A, Aghebati-Maleki L, Yousefi M. The potential of exosomes in the therapy of the cartilage and bone complications; emphasis on osteoarthritis. Life Sci. 2019;236:116861. https://doi.org/10.1016/j.lfs.2019.116861.
Shomali N, Hemmatzadeh M, Yousefzadeh Y, Soltani-Zangbar MS, Hamdi K, Mehdizadeh A, yousefi M. Exosomes: Emerging biomarkers and targets in folliculogenesis and endometriosis. J Reprod Immunol. 2020;142:103181. https://doi.org/10.1016/j.jri.2020.103181.
Cardinal-Fernández P, Ferruelo A, Esteban A, Lorente JA. Characteristics of microRNAs and their potential relevance for the diagnosis and therapy of the acute respiratory distress syndrome: from bench to bedside. Transl Res. 2016;169:102–11. https://doi.org/10.1016/j.trsl.2015.11.004.
Williams JL, Gatson NTN, Smith KM, Almad A, McTigue DM, Whitacre CC. Serum exosomes in pregnancy-associated immune modulation and neuroprotection during CNS autoimmunity. Clin Immunol. 2013;149:236–43. https://doi.org/10.1016/j.clim.2013.04.005.
De Toro J, Herschlik L, Waldner C, Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. https://doi.org/10.3389/fimmu.2015.00203.
Pillay P, Moodley K, Vatish M, Moodley J, Duarte R, Mackraj I. Exosomal Th1/Th2 cytokines in preeclampsia and HIV-positive preeclamptic women on highly active anti-retroviral therapy. Cytokine. 2020;125:154795. https://doi.org/10.1016/j.cyto.2019.154795.
James JRB, Roberts M, August Phyllis A, Bakris George. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. https://doi.org/10.1097/01.AOG.0000437382.03963.88.
de Kaminski VL, Ellwanger JH, Chies JAB. Extracellular vesicles in host-pathogen interactions and immune regulation — exosomes as emerging actors in the immunological theater of pregnancy. Heliyon. 2019;5:e02355. https://doi.org/10.1016/j.heliyon.2019.e02355.
Mitchell MD, Peiris HN, Kobayashi M, Koh YQ, Duncombe G, Illanes SE, Rice GE, Salomon C. Placental exosomes in normal and complicated pregnancy. Am J Obstet Gynecol. 2015;213:S173–81. https://doi.org/10.1016/j.ajog.2015.07.001.
Liu Z-Z, Sun G-Q, Hu X-H, Kwak-Kim J, Liao A-H. The transdifferentiation of regulatory T and Th17 cells in autoimmune/inflammatory diseases and its potential implications in pregnancy complications. Am J Reprod Immunol. 2017;78:e12657. https://doi.org/10.1111/aji.12657.
Parhizkar F, Motavalli-Khiavi R, Aghebati-Maleki L, Parhizkar Z, Pourakbari R, Kafil HS, Danaii S, Yousefi M. The impact of new immunological therapeutic strategies on recurrent miscarriage and recurrent implantation failure. Immunol Lett. 2021;236:20–30. https://doi.org/10.1016/j.imlet.2021.05.008.
Darmochwal-Kolarz D, Kludka-Sternik M, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. 2012;93:75–81. https://doi.org/10.1016/j.jri.2012.01.006.
Rahimzadeh M, Norouzian M, Arabpour F, Naderi N. Regulatory T-cells and preeclampsia: an overview of literature. Expert Rev Clin Immunol. 2016;12:209–27. https://doi.org/10.1586/1744666X.2016.1105740.
Sasaki Y, Darmochwal-Kolarz D, Suzuki D, Sakai M, Ito M, Shima T, Shiozaki A, Rolinski J, Saito S. Proportion of peripheral blood and decidual CD4+ CD25 bright regulatory T cells in pre-eclampsia. Clin Exp Immunol. 2007;149:139–45. https://doi.org/10.1111/j.1365-2249.2007.03397.x.
Hsu P, Santner-Nanan B, Dahlstrom JE, Fadia M, Chandra A, Peek M, Nanan R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am J Pathol. 2012;181:2149–60. https://doi.org/10.1016/j.ajpath.2012.08.032.
Zolfaghari MA, Motavalli R, Soltani-Zangbar MS, Parhizkar F, Danaii S, Aghebati-Maleki L, Noori M, Dolati S, Ahmadi M, SamadiKafil H, Jadidi-Niaragh F, AhmadianHeris J, Mahmoodpoor A, Hejazi MS, Yousefi M. A new approach to the preeclampsia puzzle; MicroRNA-326 in CD4+ lymphocytes might be as a potential suspect. J Reprod Immunol. 2021;145:103317. https://doi.org/10.1016/j.jri.2021.103317.
Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148:13–21. https://doi.org/10.1111/imm.12595.
Vargas-Rojas MI, Solleiro-Villavicencio H, Soto-Vega E. Th1, Th2, Th17 and Treg levels in umbilical cord blood in preeclampsia. J Matern-Fetal Neonatal Med. 2016;29:1642–5. https://doi.org/10.3109/14767058.2015.1057811.
Paeschke S, Chen F, Horn N, Fotopoulou C, Zambon-Bertoja A, Sollwedel A, Zenclussen ML, Casalis PA, Dudenhausen JW, Volk H-D, Zenclussen AC. Pre-eclampsia is not associated with changes in the levels of regulatory T Cells in peripheral blood. Am J Reprod Immunol. 2005;54:384–9. https://doi.org/10.1111/j.1600-0897.2005.00334.x.
Hu D, Chen Y, Zhang W, Wang H, Wang Z, Dong M. Alteration of peripheral CD 4 + CD 25 + regulatory T lymphocytes in pregnancy and pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:190–4. https://doi.org/10.1080/00016340701823991.
Moreno-Eutimio MA, Tovar-Rodríguez JM, Vargas-Avila K, Nieto-Velázquez NG, Frías-De-León MG, Sierra-Martinez M, Acosta-Altamirano G. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. Hindawi Com. 2014;2014:413249. https://doi.org/10.1155/2014/413249.
Cao W, Wang X, Chen T, Zhu H, Xu W, Zhao S, Cheng X, Xia L. The expression of Notch/Notch ligand, IL-35, IL-17, and Th17/Treg in preeclampsia. Dis Markers. 2015;2015:1–9. https://doi.org/10.1155/2015/316182.
Darmochwal-Kolarz D, Michalak M, Kolarz B, Przegalinska-Kalamucka M, Bojarska-Junak A, Sliwa D, Oleszczuk J. The Role of Interleukin-17, Interleukin-23, and Transforming growth factor- β in pregnancy complicated by placental insufficiency. Biomed Res Int. 2017;2017:1–5. https://doi.org/10.1155/2017/6904325.
Fu S, Zhang N, Yopp AC, Chen D, Mao M, Chen D, Zhang H, Ding Y, Bromberg JS. TGF-β induces Foxp3 + T-regulatory cells from CD4 + CD25 - precursors. Am J Transplant. 2004;4:1614–27. https://doi.org/10.1111/j.1600-6143.2004.00566.x.
Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the T H17 lineage. Nature. 2006;441:231–4. https://doi.org/10.1038/nature04754.
Peraçoli MTS, Menegon FTF, Borges VTM, de Araújo Costa RA, Thomazini-Santos IA, Peraçoli JC. Platelet aggregation and TGF-beta1 plasma levels in pregnant women with preeclampsia. J Reprod Immunol. 2008;79:84. https://doi.org/10.1016/j.jri.2008.08.001.
Feizollahzadeh S, Taheripanah R, Khani M, Farokhi B, Amani D. Promoter region polymorphisms in the transforming growth factor beta-1 (TGFβ1) gene and serum TGFβ1 concentration in preeclamptic and control Iranian women. J Reprod Immunol. 2012;94:216–21. https://doi.org/10.1016/j.jri.2012.02.006.
Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–12. https://doi.org/10.1016/j.smim.2013.10.009.
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. https://doi.org/10.1016/j.immuni.2006.01.001.
Funding
This study was supported by National Institute for Medical Research Development (NIMAD) with Grant number 982743.
Author information
Authors and Affiliations
Contributions
Mehdi Yousefi and Leili Aghebati-Maleki are principal investigators who supervised the study. Ramin Pourakbari and Forough Parhizkar participated in drafting the manuscript. Mohammad Sadegh Soltani‐Zangbar, Parisa Samadi, Majid Zamani, Roza Motavalli, Ata Mahmoodpoor, Farhad Jadidi-Niaragh, Bahman Yousefi, and Hossein Samadi Kafil were involved in data collection and conducted molecular experiments and RT-qPCR analysis; Shahla Danaii participated in patient selection and revised the draft critically. Mohammad Hojjat-Farsangi helped in the study design. Each author approved the final manuscript for submission.
Corresponding authors
Ethics declarations
Ethical Approval and Consent to Participate
This study was approved by the research ethics committee of National Institute for Medical Research Development (NIMAD) (Code: IR.NIMAD.REC.1398.073).
Consent to Participate
All participants of this article are responsible for their contributions and agree for the article to be published in this magazine.
Consent for Publication
All participants of this article are responsible for their contributions and agree for the article to be published in this magazine.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pourakbari, R., Parhizkar, F., Soltani‐Zangbar, M.S. et al. Preeclampsia-Derived Exosomes Imbalance the Activity of Th17 and Treg in PBMCs from Healthy Pregnant Women. Reprod. Sci. 30, 1186–1197 (2023). https://doi.org/10.1007/s43032-022-01059-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01059-x